For more than a decade, advocates have pushed for women to be notified if they have “dense” breasts—a factor that not only increases cancer risk but also makes tumors harder to detect on a mammogram. Finally, last month, a long-awaited U.S. Food and Drug Administration ruling determined that after giving patients a mammogram, breast imaging centers must inform them about their breast density and its associated risks—and advise them that additional imaging tests may help find cancers the mammogram could have missed.
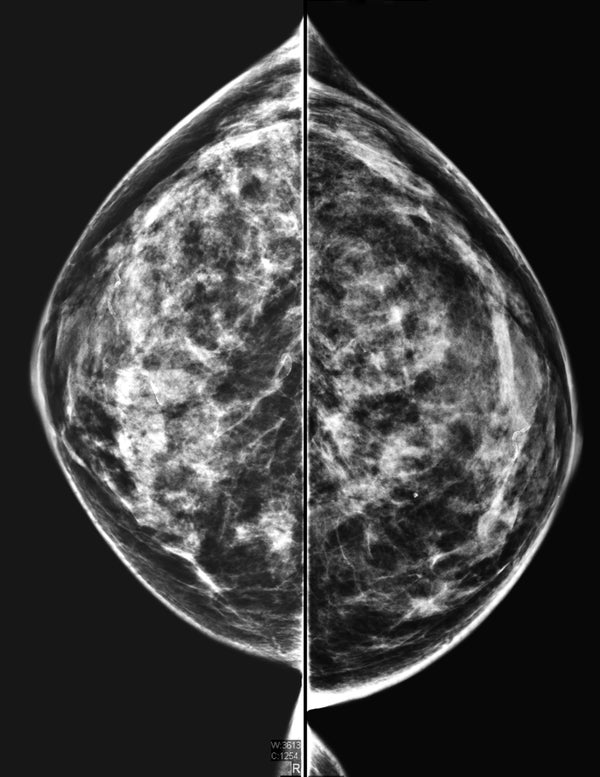
Breasts are considered dense if they are composed of more glandular and connective tissue than fat. Radiologists classify breast density in four categories: A, B, C and D. These range from extremely fatty to extremely dense, with various combinations in between. Women in categories C and D (about half of all women) are considered to have dense breasts under FDA guidelines. About 10 percent fall solely into D.
Women in category D have a twofold to fourfold greater risk of developing cancer, independent of other risk factors. By some estimates, the likelihood is higher. When density alone is considered, women in category C have a nearly average risk. Women who are transgender and take feminizing hormones can also have dense breasts, which may increase their chances of developing breast cancer somewhat. In rare cases, men can get breast cancer, but breast density isn’t generally considered a risk factor in these instances. Men who are transgender and have not undergone chest reconstruction surgery are recommended to follow the same screening protocols as women.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Women with dense breasts can develop cancers that can’t be seen on a mammogram. Both dense breast tissue and tumors appear white on a mammogram, which can mask cancers. In fatty tissue, which appears gray, white tumors are more apparent.
Advocates and physicians say the FDA mandate will result in cancers being detected more often and at earlier stages. But for an individual, determining if one needs additional screening—and how much benefit it might confer—is not so straightforward. Many doctors caution that focusing too much on density deemphasizes other factors that affect cancer risk, such as genetics, and that additional screening has a high rate of false positives.
Mammograms miss about 50 percent of cancers in women with dense breasts. Ultrasound and magnetic resonance imaging (MRI) scans have been shown to detect cancer more effectively than mammography alone. But the high cost and limited availability of MRI and debate about the benefits of additional screening for women with dense breasts have kept the tests from becoming standard in the U.S. Just last year, the European Society of Breast Imaging recommended that women aged 50 to 70 with extremely dense breasts get MRI screening, if available, every two to four years.
Currently, 38 states have laws requiring health care providers to inform people about breast density, but states vary on the degree of patient education they provide. The FDA mandate establishes consistent language for notifying patients. Breast imaging facilities have until September 2024 to comply. Only half of state laws on breast density notification mention discussing supplemental screening with a physician, and not all of them require informing patients that dense breast tissue can lead to the development of breast cancer. These inconsistencies put women in danger, says JoAnn Pushkin, a national advocate for breast density notification. Pushkin’s own cancer was missed on a mammogram 18 years ago and was later detected via ultrasound.
“Every state that enacts its own ‘inform’ law adds a new version to the mix.... This creates an inequity in actionable information a patient receives based on where she lives,” Pushkin says. “A national reporting standard is needed ... to ensure consistency from state to state.”
Know Your Risks
The FDA doesn’t specifically recommend additional screening for women with dense breasts, says the agency’s chief medical officer Hilary Marston. But the new rule advises physicians and patients to consider breast density alongside other cancer risk factors when deciding whether additional screening is necessary. “We don’t regulate the practice of medicine in any field. And in this field in particular—the follow-up conversation that really needs to happen, that we set up in this rule—is a clinician-to-patient conversation,” Marston says. “And it really needs to take into account the specifics of a patient’s case.”
To help determine a person’s cancer risk, doctors plug traits such breast density and family history into standard models, many of which are available online. According to American Cancer Society guidelines, anyone that these models deem to have at least a 20 percent lifetime risk of developing breast cancer should have an MRI in addition to a mammogram, and they may also benefit from early screening. For the models to work most effectively, women should also undergo genetic testing so health care providers can input genetic anomalies that increase cancer risk.
Women with extremely dense breast tissue and no other risk factors probably hit the 20 percent risk marker based on density alone, says Wendie Berg, a radiology professor at the University of Pittsburgh School of Medicine, who partners with Pushkin on advocacy. Women whose breasts are dense but not in the highest category “probably [have] a lifetime risk of more than 20 percent” if they have other cancer-causing risk factors, Berg says.
But because a minority of women are considered high risk based on breast density alone, many doctors caution that breast density notification laws have so far ignored other risk factors that are important for all women, wrote Jennifer Haas, now at Massachusetts General Hospital, and Celia Kaplan of the University of California, San Francisco, in a 2015 paper in JAMA Internal Medicine.
“Such laws also do not address other important risk factors for breast cancer such as age, family history of breast or ovarian cancer, BRCA and other genetic mutations, or prior breast biopsy,” Haas and Kaplan wrote. “For some women, other risk factors may be more relevant but not highlighted.”
Also, a radiologist’s determination of breast density is somewhat subjective. If radiologists fear liability for failing to report density, this could lead them to overreport it, and “supplemental screening can be ordered..., thus limiting the validity of a breast density assessment,” Haas and Kaplan added.
But advances in the past decade in both imaging and software that classifies density may help mitigate variability in assessment, the FDA wrote in its new rule. It added that “potential variability in density assessment does not outweigh the importance of communicating breast density to patients and their healthcare providers.” The connection between dense breasts and increased cancer risk has also become more apparent, Marston says. “Certainly, we knew that it could create this masking effect that could make it more difficult to identify cancers on a mammogram, but what became clear over time was that it was an independent risk factor,” she adds. “That really was what precipitated us wanting to put this into baseline standards that are available across the country.”
Choosing a Test
While additional screening for those with dense breast tissue is proven to detect far more cancers than mammography alone, it can also lead to high numbers of biopsies with false positives. This has led to significant pushback on breast density legislation.
In a study of more than 2,800 women with dense breast tissue and an increased cancer risk that was led by Berg, adding ultrasound to a mammography increased cancer detection by 55 percent. Using mammography alone, researchers found 7.6 cases of cancer per 1,000 people. Ultrasound added 4.2 cases per 1,000. In a follow-up study by Berg and her colleagues, MRI subsequently detected 14.7 more cases per 1,000—25 percent more cancer cases than mammography and ultrasound combined.
But because benign masses frequently look suspicious on ultrasound, “only about 10 percent of all the things we recommended for biopsy [after ultrasound] turned out to be cancer,” Berg says. “That’s a lot of extra testing for things that weren’t cancer, and that’s always been one of the criticisms of ultrasound.”
MRI combined with mammography is generally the most effective screening protocol for women with dense breasts because MRI is highly accurate and results in fewer false positives than ultrasound, Berg says. But MRI doesn’t eliminate the risk of false positives entirely, she adds. Several studies of MRI screening for people with dense breasts have reported false positive rates ranging from 52 to 97 per 1,000 cases.
Regardless of the chosen type of screening, many insurance companies won’t subsidize additional testing for women who don’t meet the 20 percent threshold for lifetime breast cancer risk. And many women may choose to decline MRI because of claustrophobia or other reasons. A study by Berg found that 42 percent of people who were offered a free MRI screening declined it.
Improvements in mammography may make the test more effective at detecting cancer in dense breasts. Contrast-enhanced mammography, in which an intravenously injected dye highlights areas seen in mammography imaging, has been shown to be nearly as effective as MRI. But the test is currently used primarily to assess known cancer cases. And a downside of contrast mammography is the risk of allergic reactions to the dye.
“It’s attractive because [unlike MRI machines] there’s no tunnel, and it can be done with existing equipment with usually a software upgrade,” Berg says. “Additionally, contrast-enhanced mammography may be something that can be much more widely available and probably less expensive by far than MRI because the contrast [dye] is inexpensive.”
Berg hopes the FDA’s mandate will lead to open discussion between patients with dense breasts and their doctors about how to determine individual breast cancer risk and choose the best screening.
“I think we need to educate [women] about the risks of false positives,” she says. “I think it needs to be shared decision making with her physician..., but a woman should have the information she needs to make that decision.”
Editor’s Note (4/14/23): This article was edited after posting to correct the descriptions of the percentage of women who fall under the D category of breast density and the laws requiring health care providers to inform people about breast density in 38 states.