| QUICK FACTS |
Any additional testing after a mammogram will find more things to be checked. Some may be cancer, but most will not (known as a false positive). To know whether a suspicious finding is cancer or not, further testing and possibly biopsy may be necessary. |
What is it?
Contrast-enhanced mammography (CEM) is a breast imaging technique that uses an iodine-based intravenous (IV) contrast agent in combination with a standard digital mammogram. Some cancers that are not visible on standard mammograms or tomosynthesis can be seen with the addition of contrast. The iodine-based contrast agent used is the same as the contrast agent used for CT scans, but different from the gadolinium-based contrast agents used in MRI. CEM can be used to assess the extent of cancer in women with newly diagnosed breast cancer and to monitor response to chemotherapy given prior to surgery. CEM can be used to evaluate breast symptoms or abnormalities seen on mammograms. In women recommended for screening MRI, but who are unable to tolerate or access MRI, CEM can be performed as an alternative test [1, 2]. Screening with CEM is an area of active investigation and is currently considered an “off-label use” by the United States Food and Drug Administration (FDA).
How it works
For CEM, first an IV is placed into an arm or hand vein for injection of an iodine-based contrast agent. When the contrast is injected, the patient may very briefly feel warm all over, get a metallic taste in their mouth, and then experience a sensation as if they are about to urinate. Imaging starts about two minutes after the contrast injection.
For CEM, positioning and compression of the breast are the same as for a standard digital mammogram. For each breast, two mammographic exposures (images) are obtained in each of two positions, for a total of four images per breast (eight images total for a typical CEM examination). One exposure uses low-energy x-rays and looks like a standard mammogram. The second exposure uses higher energy x-rays that are mostly absorbed by the iodine in the contrast agent. The low-energy and high-energy images are processed by the software to create an “iodine-only” image that shows only findings that “enhance.” Cancers typically enhance more than normal tissue and therefore appear brighter white while surrounding normal tissue usually appears dark (Fig. CEM-1). For this reason, most cancers are easier to see on the “iodine-only” images than on a standard mammogram or tomosynthesis. The radiologist will review the “iodine-only” images together with the low-energy images [3].
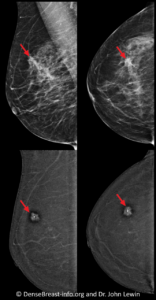
Benefits
CEM has several benefits over standard mammography, MRI, and ultrasound. CEM shows more cancers than standard mammography [3-5] or even the combination of mammography and breast ultrasound [6]. In a study of 904 women at elevated risk for breast cancer, most of whom had dense breasts, adding contrast made possible the detection of nearly 7 cancers per 1000 women screened [5]. In two studies using CEM to screen women with a personal history of breast cancer, rates of cancers detected only by addition of contrast were 17/1191 (14.3/1000 women) [7] and 9/971 (10/1000 women) [8]. Further studies are ongoing.
CEM has a very similar cancer detection rate to MRI [9, 10] and can be performed at a lower cost. CEM can be added to standard digital mammography or tomosynthesis equipment, which makes adoption easier and improves access for patients [11]. CEM is a relatively short examination, lasting about 10 minutes, which is comparable to an abbreviated MRI, and much less than the 25 minutes required for a standard breast MRI. Patients generally prefer CEM over MRI [12-14]. Unlike MRI, CEM can be used in patients with claustrophobia or metallic implants [15]. False positives (additional testing for findings that are not cancer) are less common with CEM than with MRI [9, 10]. Finally, CEM is proven to demonstrate extent of disease for both invasive ductal carcinoma and invasive lobular cancer better than standard mammography and similar to MRI [16-18].
Considerations
The radiation dose from CEM is about twice that of a standard mammogram, but still within the accepted safe range. The dose is similar to a combined 2D and 3D mammogram [19].
Iodine-based contrast agents carry some risk. Women with poor kidney function or an allergy to iodine-based contrast should not have CEM. In women over age 60 or with a history of diabetes, hypertension, or other risks for kidney disease, kidney function may need to be checked prior to injecting contrast. Mild allergic reactions, such as hives, occur in about 1% of patients within a few minutes of contrast administration. Severe allergic reactions resulting in anaphylaxis and possibly death are rare (less than 1 in 200,000). Staff must be trained in contrast reactions to provide the care needed [20].
Abnormalities seen on CEM may be recommended for additional imaging or biopsy. Some suspicious findings seen on CEM may be seen with ultrasound or additional mammogram views to allow biopsy using those methods. Some findings will require contrast-enhanced biopsy, using direct CEM guidance. If a facility does not have CEM-guided biopsy capability, MRI-guided biopsy may be needed. Lack of enhancement on CEM should not be used to avoid biopsy of suspicious calcifications visible only on the low-energy images.
Latest update 2/15/24
1. Monticciolo DL, Newell MS, Moy L, Lee CS, Destounis SV. Breast Cancer Screening for Women at Higher-than-Average Risk: Updated Recommendations from the ACR. J Am Coll Radiol 2023;20:902-914.
2. National Comprehensive Cancer Network. NCCN Guidelines. Breast cancer screening and diagnosis v.3.2023. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast- screening.pdf. Accessed January 23, 2024.
3. Lewin JM, Isaacs PK, Vance V, Larke FJ. Dual-energy contrast-enhanced digital subtraction mammography: Feasibility. Radiology 2003;229:261-268.
4. Jochelson MS, Dershaw DD, Sung JS, et al. Bilateral contrast-enhanced dual-energy digital mammography: Feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013;266:743-751.
5. Sung JS, Lebron L, Keating D, et al. Performance of dual-energy contrast-enhanced digital mammography for screening women at increased risk of breast cancer. Radiology 2019;293:81-88.
6. Sorin V, Yagil Y, Yosepovich A, et al. Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. AJR Am J Roentgenol 2018;211:W267-W274.
7. Elder K, Matheson J, Nickson C, et al. Contrast enhanced mammography in breast cancer surveillance. Breast Cancer Res Treat. 2023;199(2):221-230.
8. Gluskin J, Rossi Saccarelli C, Avendano D, et al. Contrast-Enhanced Mammography for Screening Women after Breast Conserving Surgery. Cancers (Basel) 2020;12(12):3495.
9. Pötsch N, Vatteroni G, Clauser P, Helbich TH, Baltzer PAT. Contrast-enhanced Mammography versus Contrast-enhanced Breast MRI: A Systematic Review and Meta-Analysis. Radiology 2022;305:94-103.
10. Xiang W, Rao H, Zhou L. A meta-analysis of contrast-enhanced spectral mammography versus MRI in the diagnosis of breast cancer. Thorac Cancer 2020;11:1423-1432.
11. Phillips J, Steinkeler J, Talati K, et al. Workflow considerations for incorporation of contrast-enhanced spectral mammography into a breast imaging practice.J Am Coll Radiol2018;15:881-885.
12. Hobbs MM, Taylor DB, Buzynski S, Peake RE. Contrast-enhanced spectral mammography (CESM) and contrast enhanced MRI (CEMRI): Patient preferences and tolerance.J Med Imaging Radiat Oncol2015;59:300-305.
13. Son D, Phillips J, Mehta TS, Mehta R, Brook A, Dialani VM. Patient preferences regarding use of contrast-enhanced imaging for breast cancer screening.Acad Radiol 2022;29 Suppl 1:S229-S238.
14. Berg WA, Bandos AI, Sava MG. Analytic Hierarchy Process Analysis of Patient Preferences for Contrast-Enhanced Mammography (CEM) versus MRI as Supplemental Screening Options for Breast Cancer. J Am Coll Radiol 2023;20:758-768.
15. Schiaffino S, Cozzi A, Clauser P, et al. Current use and future perspectives of contrast-enhanced mammography (CEM): a survey by the European Society of Breast Imaging (EUSOBI). Eur Radiol; Published online January 16, 2024.
16. Sumkin JH, Berg WA, Carter GJ, et al. Diagnostic Performance of MRI, Molecular Breast Imaging, and Contrast-enhanced Mammography in Women with Newly Diagnosed Breast Cancer. Radiology 2019;293:531-540.
17. Patel BK, Davis J, Ferraro C, et al. Value Added of Preoperative Contrast-Enhanced Digital Mammography in Patients With Invasive Lobular Carcinoma of the Breast. Clin Breast Cancer 2018;18:e1339-e1345.
18. Giannotti E, Van Nijnatten TJA, Chen Y, et al. The role of contrast-enhanced mammography in the preoperative evaluation of invasive lobular carcinoma of the breast, Clinical Radiology, https://doi.org/10.1016/j.crad.2024.01.035.
19. Phillips J, Mihai G, Hassonjee SE, et al. Comparative Dose of Contrast-Enhanced Spectral Mammography (CESM), Digital Mammography, and Digital Breast Tomosynthesis. AJR Am J Roentgenol 2018;211:839-846.
20. Zanardo M, Cozzi A, Trimboli RM, et al. Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): a systematic review.Insights Imaging 2019;10:76.
