| QUICK FACTS |
|
What is the Difference Between a Screening vs. Diagnostic Mammogram
A screening mammogram is performed at regular intervals to check for breast cancer when there are no signs or symptoms of disease. Screening mammograms are usually reviewed after the individual leaves the breast imaging center. Results will usually be available within a few days, and, in the USA, always within 30 days.
A diagnostic mammogram is used to check for breast cancer when there is a sign or symptom of disease, or to evaluate an abnormality seen on a screening mammogram. Common symptoms include a lump, nipple discharge, skin or nipple retraction, or a change in the size or shape of the breast. Symptoms can be due to breast cancer, but are more often due to benign (noncancerous) conditions. Breast pain, in particular, is very rarely due to cancer unless there is also a lump (and tender lumps are not usually due to cancer).
A diagnostic mammogram is monitored by the radiologist at the time of the examination. Diagnostic mammography starts with the same images as a screening mammogram and may also include additional images to review an area of concern. Ultrasound may be performed as part of diagnostic imaging at the same visit. Results of a diagnostic mammogram are given immediately to the patient.
Types of Mammography
2D Digital Mammography: The breast is placed on the surface (detector) of the mammography system and is briefly compressed between two paddles while an x-ray is taken (Fig. Mammo-1). X-rays of each breast are taken from two different positions to make sure the maximum amount of tissue is included. Occasionally additional images may be needed to fully include all the breast tissue. The total examination takes about 10 minutes. Compression spreads the breast tissue out to reduce tissue overlap and also reduces the radiation dose. Breast compression also limits motion to produce clearer images.
3D mammography (tomosynthesis): A standard 2D digital mammogram provides a 2-dimensional picture of the breast, which is a 3-dimensional object. The normal structures of the breasts (such as milk glands, fibrous tissue, fat, and blood vessels) overlap one another when viewed as a 2D image. Overlapping tissue can hide breast cancers and sometimes makes normal tissue look like breast cancer.
3D/tomosynthesis images are taken at multiple angles. The images are then reconstructed as multiple thin “slices” which can be individually scrolled through by the radiologist to review (Figs. Mammo-2, 3), like scrolling through pages of a book. Tomosynthesis can help reduce the overlap of normal tissues. This improves cancer detection and lowers the chance that normal tissue will be mistaken for cancer.
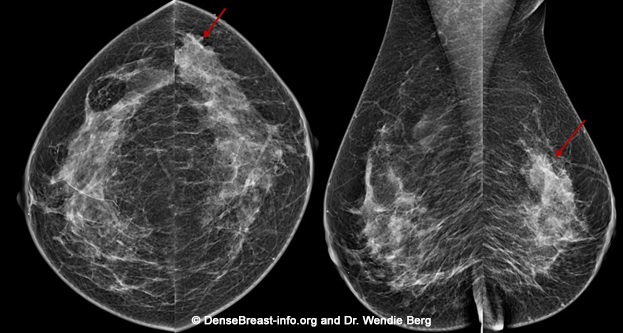
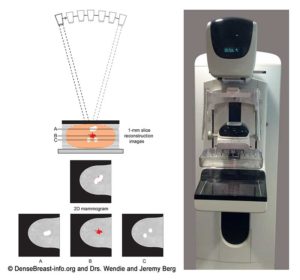
When a 2D and 3D/tomosynthesis mammogram are performed together (in “combination mode”), the radiation dose to the breast is about twice that of a 2D mammogram alone – and the dose is greater in thicker or denser breasts. This is still well below the background level of radiation from living on Earth. Many centers have the computer software needed to create a “synthetic” 2D mammogram from the same images used to create the 3D/tomosynthesis slices. This synthetic mammogram can replace the standard 2D digital mammogram so that the radiation dose from 3D/tomosynthesis is similar to a 2D digital mammogram.
Determining Breast Density
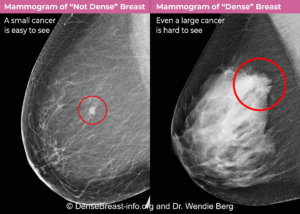
Why does breast density matter? The denser the breast tissue, the greater the risk of both developing breast cancer and of hiding cancer on a mammogram.
Radiologists have traditionally determined breast density by looking at mammogram images and comparing the amount of fibroglandular tissue (white/light) vs. fatty (gray) areas on a mammogram. Breast density is described as one of four Breast Imaging Reporting and Data System (BI-RADS®) categories: A) fatty, B) scattered fibroglandular density, C) heterogeneously dense, or D) extremely dense. Breasts that are heterogeneously dense or extremely dense are considered “dense.” Breasts with fatty or scattered fibroglandular density are “not dense”. Density assessment can also be done by computer software (see explanation of Density Assessment Software below).
In the USA, beginning September 10 2024, all mammography facilities are required to include in the patient results letter whether the breasts are dense, or not dense. In the USA and some other countries, the report sent to the referring doctor or health professional will contain the patient’s specific density category. The specific density category can be found in the medical record and formal report sent to the doctor.
Breast density is included in many breast cancer risk calculators and influences recommendations for screening in addition to mammography such as with MRI or ultrasound. Breast density may change over time and should be assessed and reported on every mammogram.
Breast Density Assessment Software
Assessment of density can vary between radiologists, especially for breasts that are on the borderline between scattered fibroglandular (“not dense”) and heterogeneously dense (“dense”) [1]. These changes in density assessment may affect what screening is recommended in addition to mammography. Modern computer programs, such as artificial intelligence, can accurately and reproducibly calculate breast density [2-5]. Computer measurements can provide more detail and more consistent results than human radiologists. Computer software can identify specific areas where a cancer may be hidden and can also predict the likelihood of developing breast cancer. These assessments can be used together with a radiologist’s assessment to improve patient care [6, 7].
Breast density assessment software is costly and is not separately reimbursed by insurance carriers, nor are radiologists required to use it. Visual density assessment and software approaches show similar performance in identifying risk of screen-detected and interval cancer [8, 9].
Mammograms and Lobular Cancer
The vast majority of invasive breast cancer is invasive ductal cancer (IDC), but about 10-15% of all breast cancer is invasive lobular cancer (ILC). ILC is especially hard to see on mammograms because it grows as single-file columns of cells and tends not to form a lump or have associated calcifications (white specks) [10]. 3D/tomosynthesis improves detection of ILC [11].
Mammography Benefits
For every 1000 individuals screened, about 5 will be found to have cancer on mammography (see Summary of Cancer Detection Rates). Screening mammography is the only breast cancer screening technology that has ever been studied in large trials, known as randomized controlled trials (RCTs), to learn if it reduces the chance of dying from breast cancer. In multiple RCTs performed from the 1960s to the 1990s, mammography screening programs reduced deaths from breast cancer by 15-22% [12]. Mammogram technology has since improved, and today mammograms reduce the risk of death from breast cancer by 40-60% in people who have them [13, 14]. The reduction in deaths is because mammograms can find cancers earlier than if a person did not get a mammogram.
Adding 3D/tomosynthesis to 2D digital mammography increases cancer detection by 1 to 2 cancers per thousand individuals screened. Several studies [15, 16] have shown there is a benefit to having 3D/tomosynthesis every year as it improves cancer detection. In studies done in the USA, 3D/tomosynthesis also reduces the number “call-backs” or “recalls” for additional testing of findings that turn out not be cancer (known as false positives)[17]. European-based studies have not shown a reduction in false positives [17, 18], likely due to low recall rates in Europe even with 2D mammograms.
Mammography Considerations
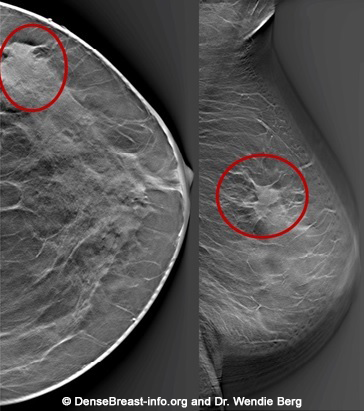
All mammograms use x-ray technology. Dense tissue absorbs more x-rays than non-dense, (fatty) tissue and appears white on a mammogram. Breast cancers are also dense and appear white. Some breast cancers are hidden (masked) on a mammogram by overlying or surrounding dense breast tissue (Fig. Mammo-4). A cancer masked on a 2D mammogram can still be masked on 3D/tomosynthesis unless the cancer is at least partially surrounded by fatty tissue. Digital 2D mammography has been shown to miss about 40% of cancers present in individuals with extremely dense breasts and 25% of cancers present in individuals with heterogeneously dense breasts [19-24]. Compared to 2D mammography, 3D/tomosynthesis improves cancer detection and reduces recalls the most on the baseline (first) screening examination, though benefits continue every year [15, 16, 25]. In individuals with extremely dense breasts, there is less benefit from 3D/tomosynthesis and cancers are often still hidden [18, 26-28].
Last update 11/17/23
1. Sprague BL, Conant EF, Onega T, et al. Variation in Mammographic Breast Density Assessments Among Radiologists in Clinical Practice: A Multicenter Observational Study. Ann Intern Med 2016; 165:457-464
2. Eriksson M, Czene K, Vachon C, Conant EF, Hall P. Long-Term Performance of an Image-Based Short-Term Risk Model for Breast Cancer. J Clin Oncol 2023:JCO2201564
3. Eriksson M, Destounis S, Czene K, et al. A risk model for digital breast tomosynthesis to predict breast cancer and guide clinical care. Sci Transl Med 2022; 14:eabn3971
4. Yala A, Lehman C, Schuster T, Portnoi T, Barzilay R. A Deep Learning Mammography-based Model for Improved Breast Cancer Risk Prediction. Radiology 2019; 292:60-66
5. Yala A, Mikhael PG, Strand F, et al. Multi-Institutional Validation of a Mammography-Based Breast Cancer Risk Model. J Clin Oncol 2022; 40:1732-1740
6. Kontos D, Winham SJ, Oustimov A, et al. Radiomic phenotypes of mammographic parenchymal complexity: Toward augmenting breast density in breast cancer risk assessment.Radiology2019; 290:41-49
7. Mainprize JG, Alonzo-Proulx O, Alshafeiy TI, Patrie JT, Harvey JA, Yaffe MJ. Prediction of cancer masking in screening mammography using density and textural features.Acad Radiol2019; 26:608-619
8. Destounis S, Johnston L, Highnam R, Arieno A, Morgan R, Chan A. Using volumetric breast density to quantify the potential masking risk of mammographic density.AJR Am J Roentgenol2017; 208:222-227
9. Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: A case-control study.Ann Intern Med2018; 168:757-765
10. Berg WA, Gutierrez L, Nessaiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR Imaging in preoperative assessment of breast cancer. Radiology 2004; 233:830-849
11. Krammer J, Stepniewski K, Kaiser CG, et al. Value of Additional Digital Breast Tomosynthesis for Preoperative Staging of Breast Cancer in Dense Breasts. Anticancer Res 2017; 37:5255-5261
12. Oeffinger KC, Fontham ET, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 Guideline update from the American Cancer Society.JAMA 2015; 314:1599-1614
13. Coldman A, Phillips N, Wilson C, et al. Pan-Canadian study of mammography screening and mortality from breast cancer.J Natl Cancer Inst 2014; 106
14. Tabar L, Dean PB, Chen TH, et al. The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening.Cancer2019; 125:515-523
15. Conant EF, Zuckerman SP, McDonald ES, et al. Five Consecutive Years of Screening with Digital Breast Tomosynthesis: Outcomes by Screening Year and Round.Radiology2020; 295:285-293
16. Bahl M, Mercaldo S, Dang PA, McCarthy AM, Lowry KP, Lehman CD. Breast cancer screening with digital breast tomosynthesis: Are initial benefits sustained?Radiology2020:191030
17. Marinovich ML, Hunter KE, Macaskill P, Houssami N. Breast Cancer Screening Using Tomosynthesis or Mammography: A Meta-analysis of Cancer Detection and Recall.J Natl Cancer Inst. 2018;110(9):942-949. doi:10.1093/jnci/djy121
18. Yi A, Chang JM, Shin SU, et al. Detection of noncalcified breast cancer in patients with extremely dense breasts using digital breast tomosynthesis compared with full-field digital mammography.Br J Radiol.2019;92(1093):20180101.
19. Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: Comparison of interval- and screen-detected cancers.J Natl Cancer Inst2000; 92:1081-1087
20. Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: A cohort study.Breast Cancer Res2017; 19:67
21. Destounis S, Johnston L, Highnam R, Arieno A, Morgan R, Chan A. Using volumetric breast density to quantify the potential masking risk of mammographic density.AJR Am J Roentgenol2017; 208:222-227
22. Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: A case-control study.Ann Intern Med2018; 168:757-765
23. Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations.Radiology2002; 225:165-175
24. Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk.JAMA2012; 307:1394-1404
25. Caumo F, Montemezzi S, Romanucci G, et al. Repeat Screening Outcomes with Digital Breast Tomosynthesis Plus Synthetic Mammography for Breast Cancer Detection: Results from the Prospective Verona Pilot Study.Radiology 2021; 298:49-57
26. Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts.JAMA2016; 315:1784-1786
27. Osteras BH, Martinsen ACT, Gullien R, Skaane P. Digital Mammography versus Breast Tomosynthesis: Impact of Breast Density on Diagnostic Performance in Population-based Screening.Radiology2019; 293(1):60-68.
28. Lowry KP, Coley RY, Miglioretti DL, et al. Screening Performance of Digital Breast Tomosynthesis vs Digital Mammography in Community Practice by Patient Age, Screening Round, and Breast Density.JAMA Netw Open. 2020;3(7):e2011792.
