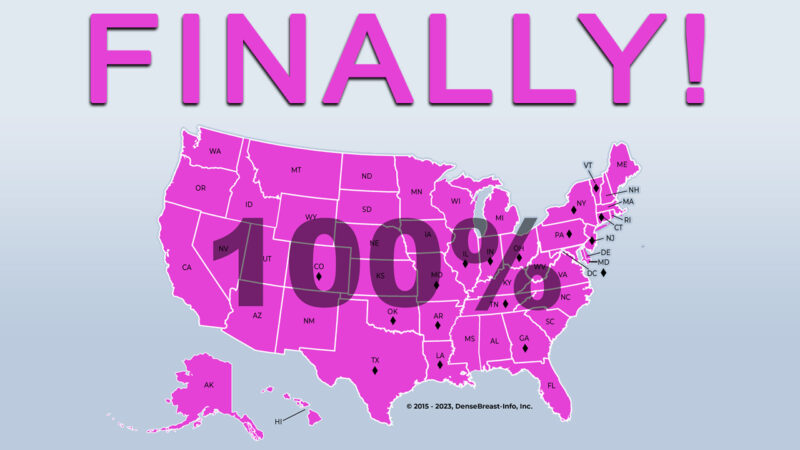
FDA National Reporting Standard
At long last, the U.S. Food and Drug Administration (FDA) has announced updates to mammography regulations to notify women and their referring providers about a woman’s breast density. States will have until Sept 10, 2024 to comply and once the rule goes into effect one of the two (not dense or dense) federal density notification statements will be required in all patient letters. More HERE.