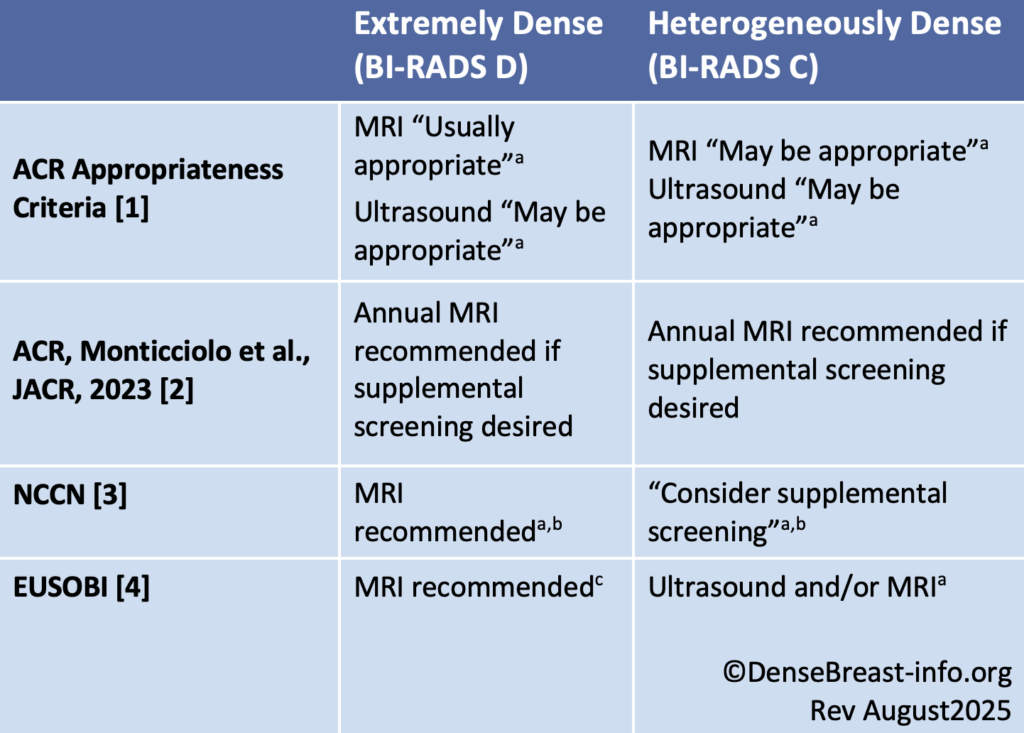
Screening guidelines for women with dense breasts and no other identifiable risk factors are provided in the table below. For screening guidelines for women with dense breasts and other risk factors, see High/Increased Risk Screening Guidelines.
Dense Breast Screening/No Other Risk Factors Guideline Comparison
Abbreviations used: ACR American College of Radiology; ACS American Cancer Society; NCCN National Comprehensive Cancer Network; EUSOBI European Society of Breast Imaginga No frequency is specified.
b Per NCCN, supplemental screening with MRI and other contrast-enhanced methods [molecular breast imaging (MBI), contrast-enhanced mammography (CEM)] have greater sensitivity than supplemental screening with ultrasound. The Society of Nuclear Medicine and Molecular Imaging (SNMMI) endorses supplemental screening with MBI after mammography in women with dense breasts at average, intermediate, or high risk; however, other screening modalities were not considered.
c EUSOBI recommends MRI in women with extremely dense breasts from ages 50-70 at least every 4 years (preferably every 2-3 years).
For evidence-based screening recommendations in any risk category designed to optimize cancer detection, see our Screening Decision Support Tool. It details:
- The American College of Radiology (ACR [2]) recommends all women, but especially Black women and women of Ashkenazi Jewish descent, undergo risk assessment and possible genetic testing by age 25.
- Those at higher risk can begin earlier and more aggressive breast cancer screening, as early as age 25.
- All women should start screening at least by age 40.
- Supplemental screening beyond mammography is not generally recommended beyond age 75. At that time, decisions about appropriate screening should be made on an individual basis depending on a woman’s overall health and risk factors.
Considerations
- If MRI screening is recommended but is not available or possible, contrast-enhanced mammography (CEM) [2,3] or molecular breast imaging (MBI) should be considered [3]. When no contrast-enhanced methods are available, screening ultrasound can be considered [2, 3].
- Per ACR, MBI is “usually not appropriate” for screening due to whole body radiation exposure [1, 2].
- CEM is currently only FDA-approved for diagnostic breast imaging in the United States and has limited availability.
- Any screening test can identify abnormalities that require additional testing for findings that are not cancer (false alarms, or “false positives”). False positives are most common the first time a test is used and decrease when prior comparison examinations are available.