The American College of Radiology (ACR [1]) recommends all women, but especially Black women and women of Ashkenazi Jewish descent, undergo risk assessment and possible genetic testing by age 25 to identify those at higher risk who can then be counseled to begin earlier and more aggressive screening for breast cancer. All women benefit most from starting screening at least by age 40. In an analysis from Harvard [2], Black, Hispanic, and Asian women have peak incidence of breast cancer in their 40s: it is especially important to start screening at least by age 40 in these groups.
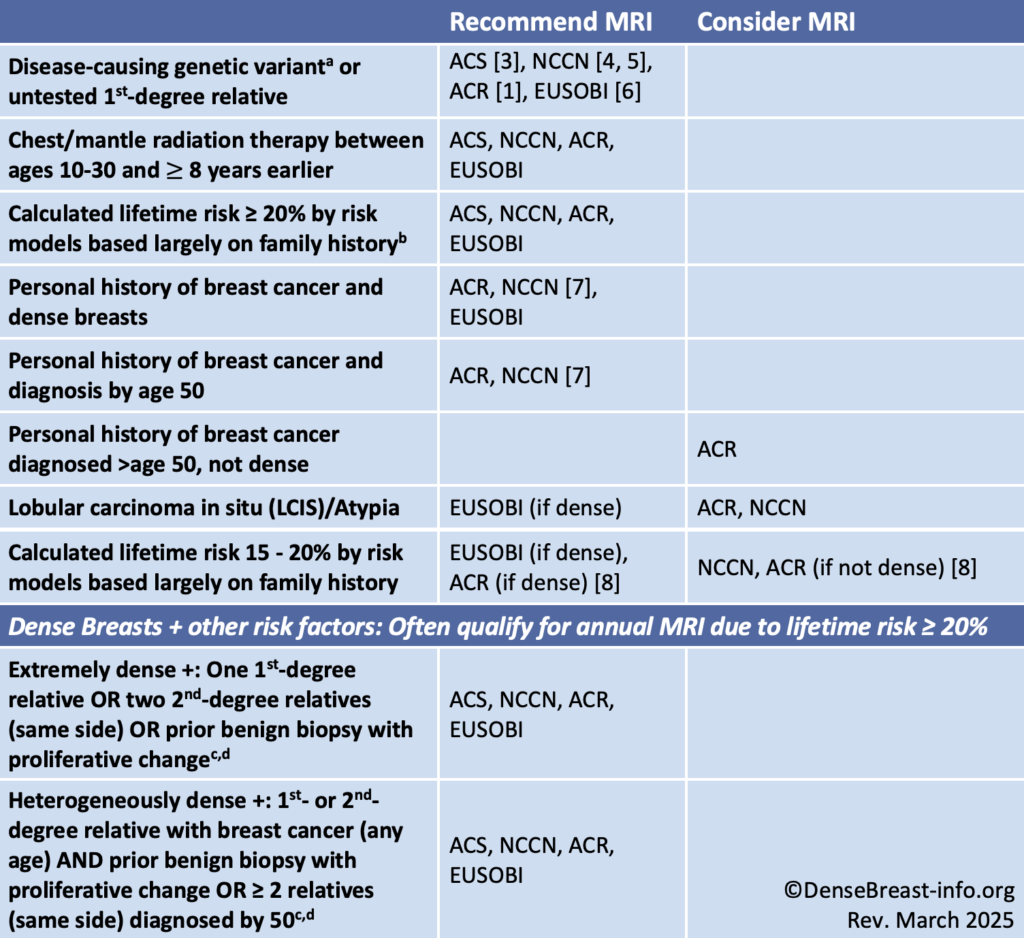
High or increased risk criteria for purposes of recommending MRI screening, shown in Table 1, differ slightly by organization. In 2007, the American Cancer Society (ACS) first suggested a threshold of 20% lifetime risk as “high risk” and reason to include yearly screening MRI, sometimes starting as early as age 25. Most women do not start screening until age 40, at which time they may be found to have dense breasts, which increase the risk of developing cancer and of having it going undetected on mammography. Individuals with dense breasts and other risk factors frequently have an estimated lifetime risk ≥20% and therefore often meet high-risk criteria for annual supplemental MRI screening. Examples are shown in the last 2 rows of Table 1. Even if formal risk assessment is not possible, imaging centers can use these guidelines to identify patients with dense breasts who meet MRI criteria. Screening guidelines for women with dense breasts and no other identifiable risk factors are provided in Table 2.
Table 1. MRI Screening, High/Increased-Risk Guideline Comparison
Abbreviations used: ACR American College of Radiology; ACS American Cancer Society; NCCN National Comprehensive Cancer Network; EUSOBI European Society of Breast Imaging
a For specific recommendations for age to start and stop screening MRI and include mammography, see https://densebreast-info.org/providers-faqs/what-is-the-screening-management-for-various-other-mutation-carriers/.
b For more about risk models, see https://densebreast-info.org/for-providers/risk-model-tutorial/.
c Lifetime risk ≥ 20% when entered into Tyrer-Cuzick risk model at https://magview.com/ibis-risk-calculator/ with otherwise average risk factors.
d Proliferative change would be listed in the pathology report from the breast biopsy as any of the following: usual ductal hyperplasia, papilloma, adenosis or sclerosing adenosis, radial scar.
What About Women with Dense Breasts and No Other Risks?
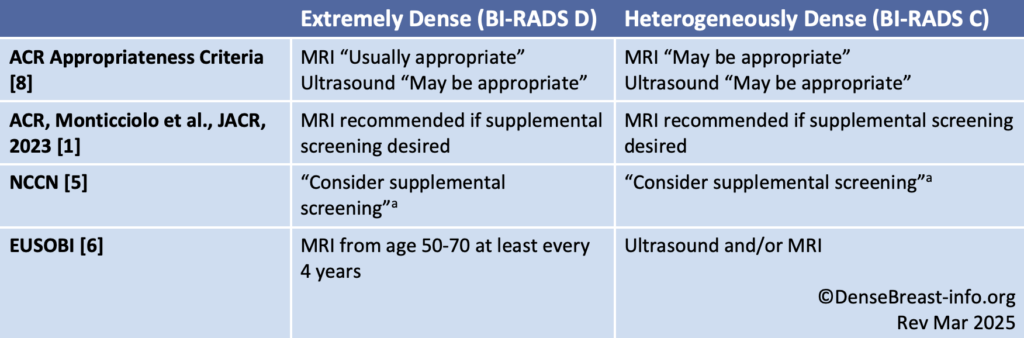
There is no single explicit guideline uniformly recommending annual supplemental MRI screening based on dense breasts alone; however, women with dense breasts and no other risk factors are at increased risk of developing breast cancer.
Table 2. Dense Breast Screening/No Other Risk Factors Guideline Comparison
Abbreviations used: ACR American College of Radiology; ACS American Cancer Society; NCCN National Comprehensive Cancer Network; EUSOBI European Society of Breast Imaging
a per NCCN, supplemental screening with MRI and other contrast-enhanced methods [molecular breast imaging (MBI), contrast-enhanced mammography (CEM)] have greater sensitivity than supplemental screening with ultrasound.
Considerations
Any screening test can identify abnormalities that require additional testing, and possibly even needle biopsy, for findings that are not cancer (false alarms, or “false positives”). False positives are most common the first time a particular test is used and decrease when there are prior comparison examinations available. The table in the Technology tab details the rates of false positives for each test.
Supplemental screening should be decided through shared decision making between an individual and their provider with consideration of the risks, benefits, desires, and availability.
For women who meet guidelines for MRI screening but who cannot access it or are unable to tolerate it (e.g. claustrophobia or metallic hardware), contrast-enhanced mammography (CEM) should be considered [1,5]. NCCN also suggests molecular breast imaging (MBI) as an alternative [5], but MBI is deemed “usually not appropriate” for screening by the ACR due to whole body radiation exposure [1,5, 8]. CEM is currently only FDA-approved for diagnostic breast imaging in the United States and has limited availability. When no contrast-enhanced methods are available, screening ultrasound can be considered [1, 5].
Supplemental screening beyond mammography is not generally recommended beyond age 75. At that time, decisions about appropriate screening should be made on an individual basis depending on a woman’s overall health and risk factors.