Health Care Provider FAQs
What Is Breast Density, Why Does It Matter?
1. What is dense breast tissue? What are dense breasts?
All breasts contain ducts, glands, fibrous tissue, and fat. Dense tissue is made of glands and fibrous tissue (“fibroglandular” tissue). Dense tissue blocks x-rays and therefore shows up as white on a mammogram. The muscle behind the breast, lymph nodes, and most other breast masses will also absorb x-rays and appear light gray or white on a mammogram, too. Fatty tissue allows more x-rays to penetrate and therefore shows up as black or dark gray on a mammogram. Each woman’s breasts are different than the next and contain a unique mix of fatty and dense tissue. Some women’s breasts are almost all fat, some have very little fat, and some are in between. Some dense breasts are mostly fibrous tissue and some are mostly glandular tissue. Dense breasts are normal and tend to become less dense with age and menopause. Breast density is not determined by how a breast looks or feels. Density is determined from a mammogram, either by a radiologist or by software.
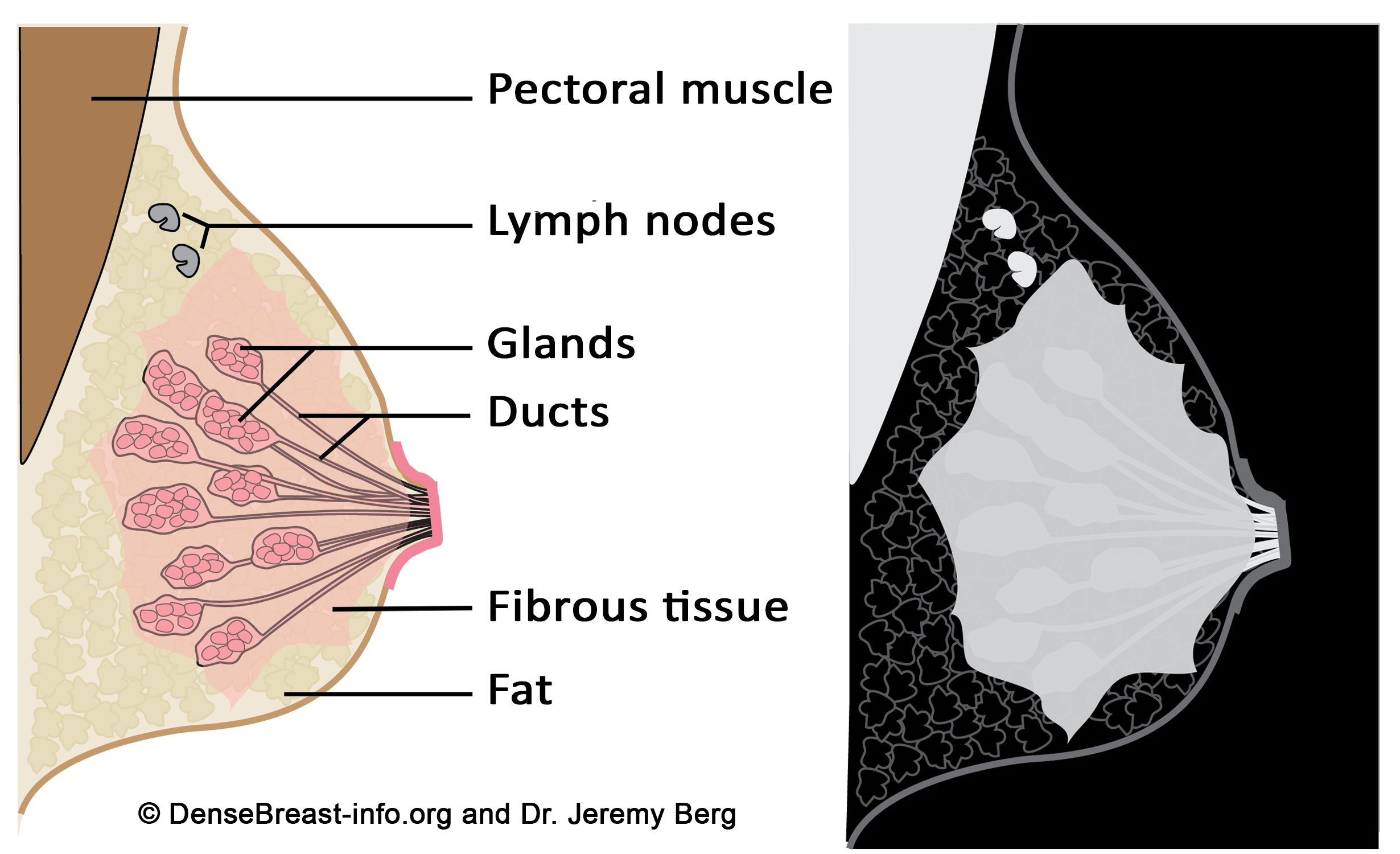
Figure Diagrams of the normal breast. Left: The normal breast is composed of milk-producing glands at the ends of ducts that lead to the nipple. Varying amounts of fibrous tissue surround the glands. There is layer of fat just beneath the skin. Often a few lymph nodes and the underlying muscle are seen near the underarm (axilla). Right: On a mammogram, fat appears dark gray, glands and fibrous tissue (dense tissue) as well as muscle and lymph nodes appear light gray or white. Masses due to cancer also appear white.
2. Are lumpy breasts or fibrocystic breasts the same as dense breasts?
Having “lumpy” breasts doesn’t mean a patient has dense breasts, nor does it mean the breasts have fibrocystic changes. Both fatty and dense breasts can feel lumpy as the ligaments that support the breast can surround fat lobules and make them feel almost like soft grapes. Breast density is not determined by how a breast looks or feels but rather by the appearance on mammography.
3. How is breast density determined?
A woman’s breast density is usually determined during her mammogram by her radiologist by visual evaluation of the images taken. Breast density can also be measured from mammograms by computer software and it can be estimated on computed tomography (CT scan) and MRI imaging. In the U.S., information about breast density is usually included in a report sent from radiologist to the referring doctor after a mammogram. Breast density information may also be included in the patient letter sent after their mammogram. In Europe, national reporting guidelines to physicians vary; no country has a public policy for density reporting to patients.
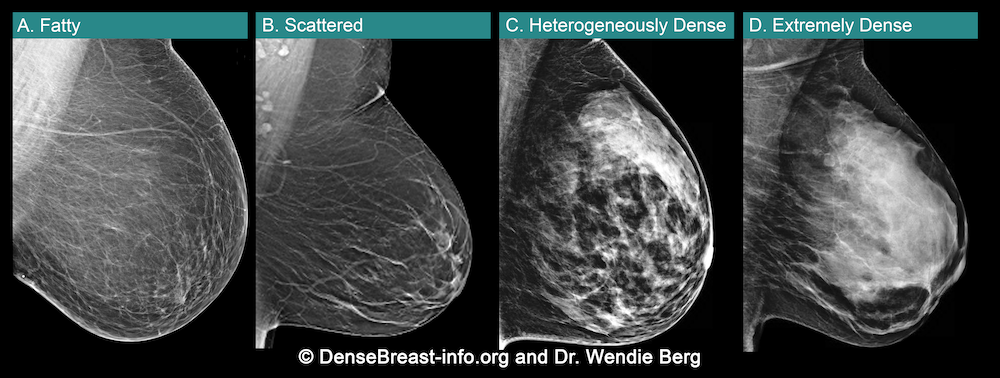
A woman’s breast tissue is categorized as one of four BI-RADS® [1] categories:
(A) Fatty; (B) Scattered fibroglandular tissue; (C) Heterogeneously dense; (D) Extremely dense
Breasts which are (C) heterogeneously dense, or (D) extremely dense, are considered “dense breasts.”
A. ALMOST ENTIRELY FATTY – On a mammogram, most of the tissue appears dark gray or black while small amounts of dense (or fibroglandular) tissue display as light grey or white.
About 10% of all women have breasts considered to be “fatty.”
B. SCATTERED FIBROGLANDULAR DENSITY – There are scattered areas of dense (fibroglandular) tissue mixed with fat. Even in breasts with scattered areas of breast tissue, cancers can sometimes be missed when they look like areas of normal tissue or are within an area of denser tissue.
About 40% of all women have breasts with scattered fibroglandular tissue.
C. HETEROGENEOUSLY DENSE – There are large portions of the breast where dense (fibroglandular) tissue could hide masses.
About 40% of all women have heterogeneously dense breasts.
D. EXTREMELY DENSE – Most of the breast appears to consist of dense (fibroglandular) tissue creating a “white out” situation, making it extremely difficult to see through.
About 10% of all women have extremely dense breasts.
References Cited
1. Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS mammography. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology, 2013
4. Why does breast density matter on a mammogram?
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. If a cancer (white) develops in an area of fat (black or dark gray), it is usually easier to detect even when it is small. Because dense tissue can hide cancers, the more fatty a breast is, the more effective the mammogram is in showing the cancer. As breast density increases, the ability to see cancer on mammography decreases. The images below are examples of how cancer presents in each breast density category:
Mammographic Images Showing How Cancer Looks in Each of the Breast Density Categories. A) A small cancer (arrow) is easily seen in a fatty breast. B) In this breast with scattered fibroglandular density, a large cancer is easily seen (arrow) in the relatively fatty portion of the breast, though a small cancer could have been hidden by areas of normal dense tissue. C) In this heterogeneously dense breast, a 4 cm cancer (arrows) is hidden by the dense breast tissue. Note the metastatic node in the left axilla (curved arrow). D) In this extremely dense breast, a cancer is seen because part of it is located in the back of the breast where there is a small amount of dark fat making it easier to see (arrow and triangle marker indicating lump). If this cancer had been located near the nipple and completely surrounded by white (dense) tissue, it probably would not have been seen on mammography.
5. Do dense breasts affect the risk of developing breast cancer?
Yes. Dense breast tissue is a risk factor for the development of breast cancer: the denser the breast, the higher the risk [1]. A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk [2].
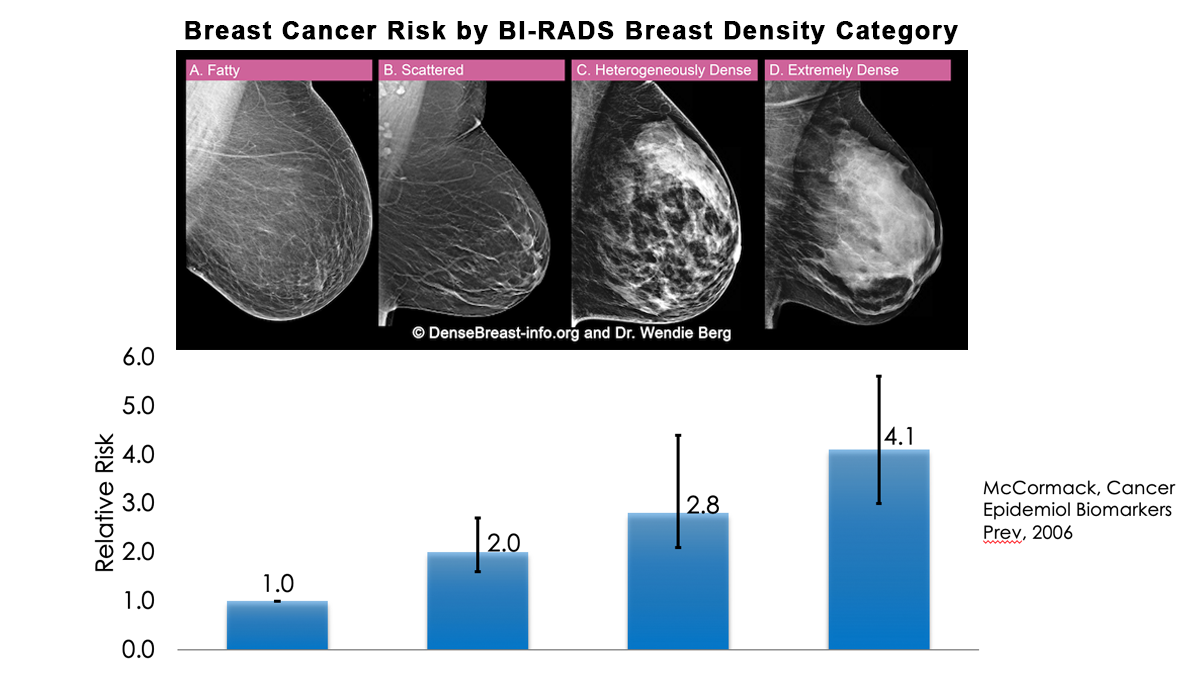
Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density [3]. In some populations, denser breasts are more common. For example, Asian women are often reported to have denser breasts than do other races; however, after accounting for age at mammography and BMI, the differences are modest [4-6]. Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue.
Risk for developing breast cancer is influenced by a combination of many different factors including age, family history of cancer (particularly breast and/or ovarian cancer), and prior atypical breast biopsies. Most women who develop breast cancer have no additional risk factors other than being female and aging.
Some risk factors can be influenced by behavior. Alcohol intake increases the risk of developing breast cancer, and the greater the intake, the greater the risk. Being overweight, especially after menopause, also increases the risk for breast cancer. Regular exercise reduces the risk of breast cancer.
There is currently no reliable way to fully know the interplay of breast density, family history, prior biopsy results, and other factors in determining overall risk. However, the largest study of its kind [7] found that dense breast tissue increases the risk of developing breast cancer more than family history, postmenopausal weight gain, or late childbearing.
For live links to breast cancer risk assessment tools click here.
References Cited
1. American Cancer Society. Breast Cancer Facts & Figures 2019-2020. Atlanta: American Cancer Society, Inc. 2019. Retrieved from https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf. Accessed April 20, 2020.
2. McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: A meta-analysis. Cancer Epidemiol Biomarkers Prev 2006; 15:1159-1169
3. Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol 2010; 28:3830-3837
4. El-Bastawissi AY, White E, Mandelson MT, Taplin S. Variation in mammographic breast density by race. Ann Epidemiol. 2001;11(4):257-63
5. del Carmen MG, Hughes KS, Halpern E, Rafferty E, Kopans D, Parisky YR, Sardi A, Esserman L, Rust S, Michaelson J. Racial differences in mammographic breast density. Cancer. 2003;98(3):590-6
6. Habel LA, Capra AM, Oestreicher N, Greendale GA, Cauley JA, Bromberger J, Crandall CJ, Gold EB, Modugno F, Salane M, Quesenberry C, Sternfeld B. Mammographic density in a multiethnic cohort. Menopause. 2007;14(5):891-9
7.. Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol 2017; 3:1228-1236
6. How does breast density compare to other risk factors for developing invasive breast cancer?
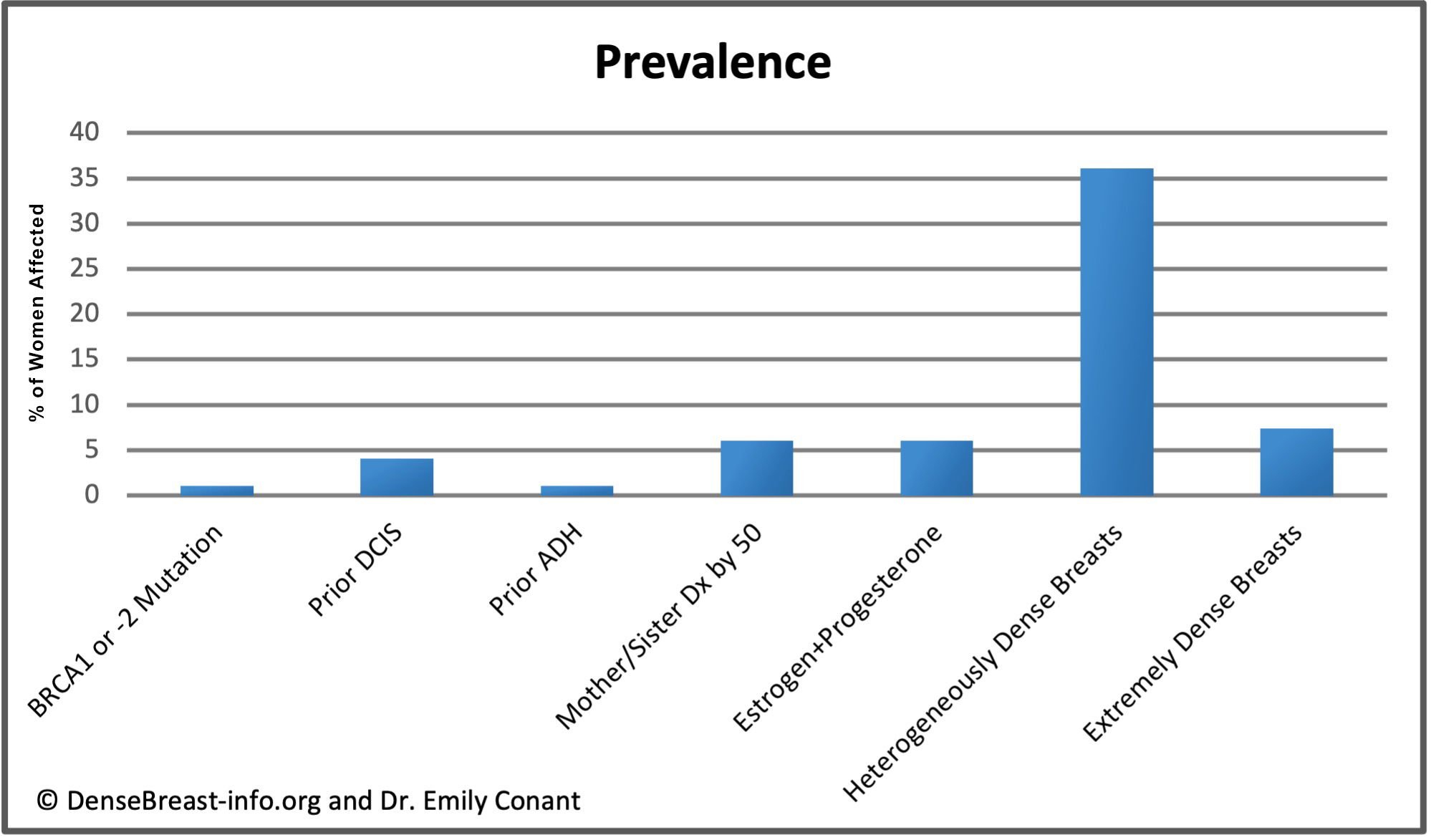
The charts below detail relative risk and prevalence [1-6].
Relative Risk: The top chart shows approximate relative risk of developing invasive breast cancer by age 80 for a woman with a given risk factor compared to a woman without that risk factor: 1) disease-causing BRCA1 or -2 mutation; 2) prior ductal carcinoma in situ; 3) prior atypical ductal hyperplasia; 4) first-degree relative (mother or sister) diagnosed with breast cancer by age 50; 5) combined estrogen and progesterone therapy after menopause; 6) heterogeneously dense breast tissue (relative to a woman with fatty breasts); or 7) extremely dense breast tissue (relative to a woman with fatty breasts).
 *The 15-year risk of developing invasive breast cancer among women with untreated DCIS (i.e. found on retrospective review of surgical biopsy specimens years later) is about 10 times greater than the risk in the general population [4, 5]. The risk 3 years or more after DCIS diagnosis in women who receive standard treatment is nearly 3 times greater than the risk in the general population [6].
*The 15-year risk of developing invasive breast cancer among women with untreated DCIS (i.e. found on retrospective review of surgical biopsy specimens years later) is about 10 times greater than the risk in the general population [4, 5]. The risk 3 years or more after DCIS diagnosis in women who receive standard treatment is nearly 3 times greater than the risk in the general population [6].
Prevalence: The lower chart shows estimated prevalence of each risk factor in American women aged 40-74, except for hormone replacement therapy which applies only to postmenopausal women. Dense breast tissue is quite common, seen in 43% of all women aged 40-74.
References Cited
1. Kuchenbaecker KB, Hopper JL, Barnes DR, Pet al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017; 317(23):2402-2416
2. Kurian AW, Hughes E, Handorf EA, et al. Breast and Ovarian Cancer Penetrance Estimates Derived From Germline Multiple-Gene Sequencing Results in Women. JCO Precis Oncol. 2017; 1:1-12
3. Hu C, Hart SN, Gnanaolivu R, et al. A Population-Based Study of Genes Previously Implicated in Breast Cancer. N Engl J Med. 2021; 384(5):440-451
4. Page DL, Dupont WD, Rogers LW, Landenberger M. lntraductal carcinoma of the breast: follow-up after biopsy only. Cancer1982; 49: 75 1-758
5. Betsill WL Jr, Rosen PP, Lieberman PH, Robbins GF. Intraductal carcinoma: Long-term follow-up after treatment by biopsy alone. JAMA1978; 239:1863-1867
6. Mannu GS, Wang Z, Broggio J, et al. Invasive breast cancer and breast cancer mortality after ductal carcinoma in situ in women attending for breast screening in England, 1988-2014: population based observational cohort study. BMJ 2020;369:m1570
7. Worsham MJ, Abrams J, Raju U, et al. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J. 2007; 13:115–21
8. Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005; 353:229–37
9. Hartmann LC, Radisky DC, Frost MH, et al. Understanding the premalignant potential of atypical hyperplasia through its natural history: a longitudinal cohort study. Cancer Prev Res (Phila) 2014; 7:211–7
10. Colditz GA, Kaphingst KA, Hankinson SE, Rosner B. Family history and risk of breast cancer: nurses’ health study. Breast Cancer Res Treat. 2012;133(3):1097-1104. doi:10.1007/s10549-012-1985-9
11. Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk [published correction appears in JAMA 2000 Nov 22-29;284(20):2597]. JAMA. 2000;283(4):485-491
12. Cummings SR, Tice JA, Bauer S, et al. Prevention of breast cancer in postmenopausal women: Approaches to estimating and reducing risk. J Natl Cancer Inst2009; 101:384-398
13. Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001; 358(9291):1389-1399
14. Couch FJ, DeShano ML, Blackwood MA, et al. BRCA1 mutations in women attending clinics that evaluate the risk of breast cancer. N Engl J Med1997; 336:1409-1415
15. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst2014; 106
What Do I Need to Know About Dense Breasts?
7. Are screening mammography outcomes different for dense breasts vs. fatty breasts?
Yes. Because cancer is more often missed on mammograms in women with dense breasts, it is more often found as a lump or because of other breast symptoms in the interval between screens (i.e., “interval cancer,” see Table 1). Such interval cancers tend to be more aggressive with worse outcomes. Cancers found in dense breasts are more often advanced (stage IIb and III), are more often multifocal or multicentric, and a mastectomy is more often needed for treatment [1]. Increasing breast density also increases the risk of recurrence in women with a history of breast cancer (especially if no radiation therapy is given) [2-5].
TABLE 1. INTERVAL CANCERS AND BREAST DENSITY [6]
| Visually Estimated Breast Density |
Odds Ratio of Interval Cancer (95% Confidence Interval) |
| < 10% | 1.0 |
| 10 to 24% | 2.1 (0.9 to 5.2) |
| 25 to 49% | 3.6 (1.5 to 8.7) |
| 50 to 74% | 5.6 (2.1 to 15.3) |
| ≥ 75% | 17.8 (4.8 to 65.9) |
References Cited
1. Arora N, King TA, Jacks LM, et al. Impact of breast density on the presenting features of malignancy. Ann Surg Oncol 2010; 17 Suppl 3:211-218
2. Eriksson L, Czene K, Rosenberg L, Humphreys K, Hall P. Possible influence of mammographic density on local and locoregional recurrence of breast cancer. Breast Cancer Res 2013; 15:R56
3. Cil T, Fishell E, Hanna W, et al. Mammographic density and the risk of breast cancer recurrence after breast-conserving surgery. Cancer 2009; 115:5780-5787
4. Huang YS, Chen JL, Huang CS, et al. High mammographic breast density predicts locoregional recurrence after modified radical mastectomy for invasive breast cancer: A case-control study. Breast Cancer Res 2016; 18:120
5. Lowry KP, Braunstein LZ, Economopoulos KP, et al. Predictors of surveillance mammography outcomes in women with a personal history of breast cancer. Breast Cancer Res Treat 2018; 171:209-215
6. Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007; 356:227-236
8. Is it unusual to have dense breasts?
No. Dense breasts are neither unusual nor abnormal. By age:
- More than half of women under the age of 50 have dense breasts.
- About 40 percent of women in their 50s have dense breasts.
- About 25 percent of women age 60 and older have dense breasts [1, 2].
Generally, glandular tissue (which contributes to breast density) tends to shrink after menopause so that sometimes the breasts will appear less dense on mammograms as a woman gets older. However, many women continue to have dense breast tissue after menopause. During pregnancy and breastfeeding, the dense tissue grows and the breasts become denser and often larger.
The tissue composition of every breast is different (and can differ during a woman’s own lifetime) which is why women should know their own breast density and understand the limitations of mammography for their breast type.
References Cited
1. Kerlikowske K, Ichikawa L, Miglioretti DL, et al. Longitudinal measurement of clinical mammographic breast density to improve estimation of breast cancer risk. J Natl Cancer Inst 2007; 99:386-395
2. Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst 2014; 106
9. Is breast size related to breast density?
Smaller breasts tend to be dense, and large breasts are more often relatively fatty, but there is wide variation at the individual level.
10. If a woman has dense breasts, will she always?
Breasts tend to become less dense as women get older (see Figure below), especially after menopause, as the glandular tissue atrophies and the breast may appear more fatty-replaced. Taking hormones for menopausal symptoms can delay the regression of dense tissue. If a patient loses a lot of weight, her breasts may appear denser due to the relative loss of fat. There is also variability in the visual assessment of breast density so that the density reported in the mammogram might be “scattered” one year and “heterogeneously dense” the next year or vice versa without any true change in breast density. In both situations, there are areas within the breast where there is some dense tissue which could mask cancer detection. In recent years, the use of automated computer-based density assessment can provide reproducible and objective quantification of breast density, avoiding inter- and intraobserver variability [1].
References Cited
1. Destounis S, Arieno A, Morgan R, Roberts C, Chan A. Qualitative versus quantitative mammographic breast density assessment: Applications for the US and abroad. Diagnostics (Basel) 2017; 7
11. If a woman does not have dense breasts, what should she do?
Annual mammography (with tomosynthesis if available) is recommended if she is over the age of 40 and in good health. If the patient is at high risk of developing breast cancer, she may be recommended to have an MRI every year in addition to mammography.
12. Does having dense breasts increase the chance of dying from breast cancer?
Though there is not extensive research on this topic, one study [1] indicated that because women with dense breasts are at a greater risk of developing breast cancer, their risk of dying from breast cancer is about double that of the general population. Two other studies evaluated women with breast cancer and found an increased risk of death among women with fatty breasts; the reasons for this are not well understood [2, 3]. A recent analysis from The Netherlands showed a smaller estimated mortality reduction from screening mammography of 13% in women with dense breasts compared to 41% in women with fatty breasts. Reduced benefit from mammographic screening is attributed to the masking effect of dense tissue with tumors detected later, when they were larger, in women with dense breasts [4].
References Cited
1. Chiu SY, Duffy S, Yen AM, Tabar L, Smith RA, Chen HH. Effect of baseline breast density on breast cancer incidence, stage, mortality, and screening parameters: 25-year follow-up of a Swedish mammographic screening. Cancer Epidemiol Biomarkers Prev 2010; 19:1219-1228
2. Gierach GL, Ichikawa L, Kerlikowske K, et al. Relationship between mammographic density and breast cancer death in the breast cancer surveillance consortium. J Natl Cancer Inst 2012; 104:1218-1227
3. Masarwah A, Auvinen P, Sudah M, et al. Very low mammographic breast density predicts poorer outcome in patients with invasive breast cancer. European radiology 2015; 25
4. van der Waal D, Ripping TM, Verbeek AL, Broeders MJ. Breast cancer screening effect across breast density strata: A case-control study. Int J Cancer 2017; 140:41-49
Mammograms and Dense Breasts
13. If a mammography report indicates the patient has heterogeneously dense or extremely dense tissue but is otherwise categorized as “negative” or “benign” what should be considered next?
Dense breasts are “normal.” In fact, 40% of women over age 40 have dense breasts. But dense breast tissue can hide cancer on a mammogram and can reduce the effectiveness of mammography screening. So a “normal,” “negative,” or “benign” mammogram result does not reliably exclude cancer in women with dense breasts. This is why, sometimes, a woman with dense breasts may have cancer detected soon after a “normal,” “negative,” or “benign” mammogram. This is known as an “interval cancer.” To find cancer in a woman with dense breasts, additional screening should be considered. See European Screening Decision Support Tool (or, in the U.S.: Flow Chart: Who Needs More Screening?).
14. A patient recently had a “normal” mammogram and has extremely dense breasts. She now feels a lump. What should you recommend?
It is important for any woman not to ignore a lump just because the recent mammogram was normal, and this is especially important if the breasts are dense. While cysts, other benign masses, and areas of normal tissue can present as lumps, malignant masses, especially those lacking calcifications, are frequently masked by dense breast tissue and a “normal,” “negative,” or “benign” mammogram does not mean that there is no cancer present. Tomosynthesis can help show some cancers not found with 2D-mammography, but ultrasound is the test of choice to evaluate palpable lumps and allows targeted assessment and correlation of the area being felt with findings on ultrasound. If there is a mass suspicious for cancer, the radiologist/technologist may also include ultrasound of the tissue in the axilla (under the arm) because the first place cancer will spread is to lymph nodes in the axilla. Cancers presenting because of symptoms prior to the next annual mammogram are called “interval cancers” and interval cancers are increasingly common with increasing breast density.
15. When should screening mammography begin and stop?
Based on randomized trials (invitation to screening) mammography, there is at least a 15% decrease in deaths due to breast cancer in women screened in their 40s and a 22% reduction in deaths among women screened from ages 50 to 74 [1]. In observational studies of women actually having mammographic screening, reduction in deaths due to breast cancer is closer to 40% [2, 3]. Based on these results, the American College of Radiology (ACR) recommends annual screening beginning at age 40 for women at average risk for breast cancer [4]. The European Society of Breast Imaging (EUSOBI) recommends biennial screening mammography for average-risk women aged 50–69 years; extension up to 73 or 75 years biennially and from 40–45 to 49 years, annually [5]. Breast cancer incidence peaks earlier, in the 40s, for African American, Asian, and Hispanic women [6], than for Caucasian women when the peak is in the early 50s. As such, it is especially important for African American, Asian, and Hispanic women to start screening by age 40. The age to stop screening should be based on a woman’s overall health status. Women with a life expectancy less than 5-7 years are unlikely to realize a benefit from screening mammography.
Women at high risk for breast cancer, because of known or suspected disease-causing mutations in BRCA or other genes such as P53, should begin screening earlier; American Cancer Society recommendations, as well as those of the European Society of Mastology (EUSOMA), include annual MRI [7, 8]: BRCA-1 carriers should begin by age 25, and BRCA-2 carriers by age 30. Women with a history of radiation therapy to the chest (e.g., for Hodgkin’s disease) before age 30 should begin screening with mammography and MRI at age 25, or 8 years after treatment, whichever is later. Emerging evidence suggests that for BRCA-1 carriers who have annual MRI, the benefit of mammography is relatively small before age 40 [9]. Once a woman has had breast cancer, she should have at least annual mammography; if she also has dense breasts and/or was diagnosed with breast cancer by the age of 50, the ACR recommends she consider annual MRI in addition to mammography [10].
For other women with a family history of breast cancer, it is important to consider many factors, including the age at diagnosis of family members. Several risk models have been developed. While all models can over- or underestimate risk in a given individual, the Tyrer-Cuzick model is the most consistently accurate and, as of September 2017, includes breast density as a risk factor. For women estimated to have a lifetime risk of breast cancer of 20% or more, annual MRI screening has been recommended in addition to mammography [7, 10].
References Cited
1. Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: An update for the U.S. Preventive Services Task Force. Ann Intern Med 2009; 151:727-737, W237-742
2. Coldman A, Phillips N, Wilson C, et al. Pan-Canadian study of mammography screening and mortality from breast cancer. J Natl Cancer Inst 2014; 106
3. Broeders M, Moss S, Nystrom L, et al. The impact of mammographic screening on breast cancer mortality in Europe: A review of observational studies. J Med Screen 2012; 19 Suppl 1:14-25
4. Monticciolo DL, Newell MS, Hendrick RE, et al. Breast cancer screening for average-risk women: Recommendations from the ACR Commission on Breast Imaging. J Am Coll Radiol 2017; 14:1137-1143
5. Sardanelli F, Aase HS, Alvarez M, et al. Position paper on screening for breast cancer by the European Society of Breast Imaging (EUSOBI) and 30 national breast radiology bodies from Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Israel, Lithuania, Moldova, The Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Spain, Sweden, Switzerland and Turkey. Eur Radiol 2017; 27:2737-2743
6. Stapleton SM, Oseni TO, Bababekov YJ, Hung YC, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg 2018; 153:594-595
7. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75-89
8. Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur J Cancer 2010; 46:1296-1316
9. Heijnsdijk EA, Warner E, Gilbert FJ, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI screening-MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomarkers Prev 2012; 21:1458-1468
10. Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: Recommendations from the ACR. J Am Coll Radiol 2018; 15:408-414
16. Should mammography screening begin at age 40 or 50?
Women should begin screening at age 40. Though breast cancer is more common as women get older, it is still important to begin screening at 40 because:
- We screen for breast cancer to find it EARLY, when it is easier to treat and most survivable.
- Breast cancer is the number one cause of death in women aged 35 to 54 years.
- Deaths from breast cancer are reduced the most when screening starts at age 40. The most years of life are saved when screening starts at age 40.
- More than half of women in their 40s have dense breasts (heterogeneously dense or extremely dense). Dense breast tissue increases the risk for developing breast cancer and the consideration of additional screening after a mammogram.
- Women at “high risk” for breast cancer, most often because they have a disease-causing mutation (such as BRCA1 or BRCA2), should begin screening even younger – at least by age 30 and with the inclusion of an MRI.
WHAT ABOUT FALSE ALARMS (KNOWN AS “FALSE POSITIVES”)?
- About 10% of women having a screening mammogram will be called back (recalled) for extra testing or views. THIS IS NORMAL. Among women called back, 95% do not have cancer. If a needle biopsy is necessary, even that is a simple test not much different from a dental filling.
- The newer technique of 3D-mammography (also known as tomosynthesis), is better able to show cancer AND results in fewer callbacks for extra testing.
WHAT ABOUT SCREENING IN DENSE BREASTS?
- Younger women are more likely to have dense breast tissue that can hide cancer on mammography.
- In women who have breasts categorized as “dense” (heterogeneously dense or extremely dense), adding screening ultrasound after a mammogram can help find more breast cancers. However, ultrasound also finds areas/masses that are not cancer and increases the chance of needing a needle biopsy to determine if such areas/masses detected are cancerous or not.
IS IT COVERED?
- Under the Affordable Care Act, insurance carriers are required to cover the full cost of screening mammography. If the screening is performed by 3D mammography (tomosynthesis), the full cost might not be covered by some insurance companies in some states.
- Insurance coverage for additional screening tests, such as ultrasound or MRI, varies by state and by insurance company. Women should check with their insurance carriers to determine how additional tests will be covered. In women at high risk for breast cancer, most insurers will cover screening MRI (regardless of density) though a deductible/co-pay will typically apply, and pre-authorization may be needed.
- Diagnostic mammography is performed to evaluate abnormalities found on screening or when a woman has signs or symptoms of breast cancer. A deductible/co-pay will usually apply for diagnostic mammography.
17. What is “risk-based mammography screening” for women age 40-49? Is it safe?
The idea that only women at increased risk should be screened in their 40s is an example of “risk-based screening.”
Since breast cancer is less common in the 40s than in older women, there are fewer cancers to be found. At the same time, largely because it may be the first time a woman has mammography screening, and also because the breasts are more often dense, there are more false positives (callbacks for additional testing when no cancer is found) in women in their 40s. In risk-based screening, the goal is to reduce the number of women who are screened or the frequency of screening or both.
However, if mammography screening were limited to only women with family history of breast cancer and/or dense breasts, most studies suggest that the majority of women diagnosed with cancer in their 40s would not be screened.
Several studies have examined how many cancers occur in women who would not be screened by “risk-based screening.” In the study of Destounis et al [1], 61% of women diagnosed with cancer in their 40s had no family history of breast cancer. In the study of Price et al [2], only 24% of women diagnosed with breast cancer in their 40s had a very strong family history of breast cancer (first degree relative diagnosed by age 50 or two first-degree relatives diagnosed) or extremely dense breasts. Some family history of breast cancer (first-degree relative diagnosed at any age) or dense breast tissue (including heterogeneously dense breast tissue) was present in 79% of women diagnosed with breast cancer. Neal et al [3] found only 20% of women diagnosed with breast cancer in their 40s had a family history of breast cancer.
The ongoing WISDOM Study [4] (Women Informed to Screen Depending On Measures of risk) randomizes consenting women to “adaptive screening.” In women who accept randomization, screening will commence for a woman in her 40s only when she has 5-year risk at least as high as an average 50-year-old woman (1.3% 5-year risk of developing breast cancer) and screening would then be biennial for most women and annual for women with extremely dense breasts or other risks. Burnside et al [5] evaluated cancer yield if screening were limited to those women in their 40s who had at least the same 5-year risk of breast cancer as a woman of age 50. They found that only 13/50 (26%) of cancers occurred in women who would have been screened based on risk. If screening were limited to women over age 45, 34/50 (68%) of cancers diagnosed in women in their 40s would have been found on screening.
Importantly:
- Breast cancer is the number one cause of death in women aged 35 to 54 years.
- Mammography has been proven to reduce deaths due to breast cancer in women screened beginning at age 40.
- Treatment is less invasive for women diagnosed by screening, including women diagnosed in their 40s [6].
- 25% of all years of life lost to breast cancer occur in women diagnosed before the age of 45.
Thus, under any scenario of restricting mammography screening in the 40s to only those women at increased risk, the cancer-detection benefits of screening are reduced.
All U.S. national medical societies agree that breast cancer screening beginning at the age of 40 saves the most lives. For more information about screening guidelines by medical society, click here.
References Cited
1. Destounis SV, Arieno AL, Morgan RC, et al. Comparison of breast cancers diagnosed in screening patients in their 40s with and without family history of breast cancer in a community outpatient facility. AJR Am J Roentgenol 2014; 202:928-932
2. Price ER, Keedy AW, Gidwaney R, Sickles EA, Joe BN. The potential impact of risk-based screening mammography in women 40-49 years old. AJR Am J Roentgenol 2015; 205:1360-1364
3. Neal CH, Rahman WT, Joe AI, Noroozian M, Pinsky RW, Helvie MA. Harms of restrictive risk-based mammographic breast cancer screening. AJR Am J Roentgenol 2018; 210:228-234
4. Esserman LJ, Study W, Athena I. The WISDOM Study: Breaking the deadlock in the breast cancer screening debate. NPJ Breast Cancer 2017; 3:34
5. Burnside ES, Trentham-Dietz A, Shafer CM, et al. Age-based versus risk-based mammography screening in women 40-49 years old: A cross-sectional study. Radiology 2019:181651
6. Ahn S, Wooster M, Valente C, et al. Impact of screening mammography on treatment in women diagnosed with breast cancer. Ann Surg Oncol 2018; 25:2979-2986
18. Should women with dense breasts still have mammography screening?
Yes. Mammography is the first step in screening for most women. While additional screening may be recommended for women with dense breasts and/or high risk for developing breast cancer, there are still some cancers and precancerous changes that will show on a mammogram better than on ultrasound or MRI. Whenever possible, women with dense breasts should have digital mammography rather than film mammography, and preferably with DBT (tomosynthesis) due to slightly improved cancer detection using digital mammography [1]. About half of cancers seen on mammography have calcifications (white dots like salt crystals), and calcifications can be seen even in dense areas of the breast (Figure below). It is important to know that at least a few calcifications can be seen in nearly all breasts and that the vast majority of calcifications seen on a mammogram are not due to cancer. Some calcifications require special magnification mammography views to be adequately evaluated. A biopsy may be recommended for calcifications which are new or increasing and have a concerning appearance on magnification views. Even when a biopsy is recommended for calcifications, only about 1 in 4 or 5 are shown to be due to cancer. When there are no calcifications, some masses due to cancer can be seen in dense breasts because they distort (pucker) the tissue around them. Some masses due to cancer are seen in dense breasts because at least a portion of the mass is in an area where the breast is more fatty.
Women who are pregnant may want to defer screening mammography until after the pregnancy. (For women who are pregnant with a lump or other breast problem, diagnostic ultrasound is performed first, and mammography can be used safely and effectively performed if needed with abdominal shield protection.) For women who are breastfeeding, if they will only be nursing for a short time (3-5 months), they might defer screening mammography until 2-3 months after the baby is weaned. For those planning on breastfeeding 6 months or longer, mammography screening can be performed while nursing but probably best to schedule it after the first 3 months when the breasts are less likely to be engorged. Patients who are breastfeeding will almost certainly have dense breasts during that time.
Mammography Shows Some Early Breast Cancers Not Seen on Ultrasound. Magnification of mammographic images of heterogeneously dense breasts show new grouped calcifications (white specks like salt crystals within yellow circles). These are difficult or impossible to see with ultrasound. Most calcifications seen on mammograms are not due to cancer; however, biopsy showed these to be due to ductal carcinoma in situ (DCIS), a noninvasive cancerous change which, if left untreated, can progress to invasive breast cancer.
References Cited
1. Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 2005; 353:1773-1783
19. Can a patient skip mammography and instead have a screening ultrasound?
Ultrasound should NOT be considered a replacement or substitute for mammography, as many breast cancers (about half of DCIS, seen most often as calcifications, and one in four to five invasive breast cancers) may only be depicted on mammography/tomosynthesis, even in women with dense breasts. Ultrasound screening should only be done as an adjunct to screening mammography in patients with dense breast tissue.
Women who refuse mammography based on concerns about radiation or other factors (for example, pain/discomfort from compression) should be counseled on the safety of mammography, the low risks of the radiation associated with mammography, and the success of mammography as a screening test. Many centers will not perform screening ultrasound without a screening mammogram/tomosynthesis.
However, in some uncommon situations, ultrasound may be performed as the primary test for screening if the center has the required expertise:
- The most common situation for using ultrasound as a primary screening tool would be in young patients (under age 30) who are at high risk for developing breast cancer but who are unable to have breast MRI due to pregnancy or other factors.
- Women for whom mammography cannot be performed for any reason may benefit from ultrasound screening, such as women over age 40 with disability impacting the ability to cooperate with mammographic positioning.
In women at elevated risk of breast cancer who cannot have MRI due to implanted devices, claustrophobia, allergy to contrast (gadolinium chelate), or body habitus precluding positioning, ultrasound screening can also be used as a supplement to mammography.
20. If a woman is a breast cancer survivor and has dense breasts, is mammography adequate screening?
Often not. Across all breast densities, compared to women without a history of breast cancer, mammography is less sensitive and interval cancer rates are higher in women who have had breast cancer [1]. Women diagnosed before the age of 50 or with dense breasts are especially likely to have subsequent breast cancer missed by screening mammography [2]. In these high-risk women, early detection of second breast cancers has been shown to improve survival [3], so there are guidelines recommending additional screening beyond mammography.
Tomosynthesis, or 3D mammography, improves cancer detection in most women (by 1-2 per 1000 screening examinations) compared to standard 2D mammography. In all women with dense breasts, however, tomosynthesis remains limited, as cancers that lack calcifications can be masked by overlying tissue. For women with dense breasts and prior breast cancer, the American College of Radiology now recommends [4] annual MRI in addition to either 2D or 3D mammography for the following women (provided the patient has not had bilateral mastectomy):
- All women with a personal history of breast cancer and dense breasts.
- Women with any breast density diagnosed with breast cancer by age 50.
Women with a personal history of breast cancer diagnosed after age 50 even with breasts that are not dense should strongly consider supplemental screening with MRI, especially if other risk factors are present.
If breast MRI is not possible, contrast-enhanced mammography (CEM) or molecular breast imaging (MBI) are other options. Ultrasound can be considered if these options are not possible.
What are the data?
Several supplemental screening methods improve detection of early, node-negative invasive breast cancers in women with a history of breast cancer. MRI will show the most cancers, even after combined mammography (and several studies also used ultrasound), at an average of 15 per 1000 examinations [5-11]. If MRI is not possible, contrast-enhanced mammography may be considered, which improves cancer detection in women who have undergone breast conservation over digital mammography (15 vs. 6 per 1000 examinations, respectively) [12]. If these options are not possible or not available, screening ultrasound can be added to annual mammography, though the added cancer detection (at 2-4 per 1000 examinations) is less than that achieved with MRI or CEM. Ultrasound is not indicated for screening women who have had screening MRI [13].
References Cited
- Houssami N, Abraham LA, Miglioretti DL, et al. Accuracy and outcomes of screening mammography in women with a personal history of early-stage breast cancer. JAMA 2011; 305:790-799
- Lowry KP, Braunstein LZ, Economopoulos KP, et al. Predictors of surveillance mammography outcomes in women with a personal history of breast cancer. Breast Cancer Res Treat 2018; 171:209-215
- Houssami N, Ciatto S, Martinelli F, Bonardi R, Duffy SW. Early detection of second breast cancers improves prognosis in breast cancer survivors. Ann Oncol. 2009; 20(9):1505-10
- Monticciolo DL, Newell MS, Moy L, Lee CS, Destounis SV. Breast cancer screening for women at higher-than-average risk: Updated recommendations from the ACR. J Am Coll Radiol2023; S1546-1440(23)00334-4
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012; 307(13):1394-404
- Giess CS, Poole PS, Chikarmane SA, Sippo DA, Birdwell RL. Screening Breast MRI in Patients Previously Treated for Breast Cancer: Diagnostic Yield for Cancer and Abnormal Interpretation Rate. Acad Radiol. 2015; 22(11):1331-7
- Gweon HM, Cho N, Han W, et al. Breast MR imaging screening in women with a history of breast conservation therapy. Radiology. 2014; 272(2):366-73
- Weinstock C, Campassi C, Goloubeva O, et al. Breast magnetic resonance imaging (MRI) surveillance in breast cancer survivors. SpringerPlus. 2015; 4:459
- Lehman CD, Lee JM, DeMartini WB, et al. Screening MRI in Women With a Personal History of Breast Cancer. J Natl Cancer Inst. 2016; 108(3)
- Nadler M, Al-Attar H, Warner E, et al. MRI surveillance for women with dense breasts and a previous breast cancer and/or high risk lesion. Breast. 2017; 34:77-82
- Vreemann S, Gubern-Merida A, Schlooz-Vries MS, et al. Influence of Risk Category and Screening Round on the Performance of an MR Imaging and Mammography Screening Program in Carriers of the BRCA Mutation and Other Women at Increased Risk. Radiology. 2018; 286(2):443-51
- Gluskin J, Rossi Saccarelli C, Avendano D, et al. Contrast-Enhanced Mammography for Screening Women after Breast Conserving Surgery. Cancers (Basel) 2020; 12:3495
- Berg WA. Data Do Not Support Semiannual Screening US after MRI, and Screening Mammography after MRI Has Limited Benefit. Radiology 2023; 307:e230932
21. Are a digital mammogram and a 3D mammogram (known as tomosynthesis) the same thing?
No, but they are different types of mammograms. Both involve computer-generated images. A standard 2D digital mammogram captures images from two different angles (or views). Tomosynthesis (3D) captures images from many different angles (projection images). The multiple images are then compiled by a computer and used to create thin “slice” images of a breast. A “3D” mammogram can be performed in addition to a standard 2D mammogram. Tomosynthesis uses x-rays that produce about the same radiation exposure to the breasts as a standard mammogram: if a patient has both 2D and 3D their breasts will receive nearly twice the amount of radiation as from a standard mammogram, though the combined dose is still within standard safety limits. A new technique that creates a 2D-like image from the projection images, i.e. a “synthetic” 2D image, is being used in many centers instead of the standard 2D mammogram. The total radiation dose from tomosynthesis with synthetic 2D images is similar to or slightly more than a standard 2D mammogram and varies with breast thickness and tissue density.
22. Is the mammography recall rate (or the false positive rate/false alarm rate) higher for women with dense breasts than in women with fatty breasts?
Yes. Women with dense breasts are about twice as likely to be called back for additional testing as are women with non-dense breasts, and the vast majority (about 95%) of callbacks will not show cancer [1, 2]. Thus, the denser the breast, the more likely a false positive (additional testing when no cancer is present) is to occur. Women with extremely dense breasts are about twice as likely to experience a false positive as are women with fatty breasts.
References Cited
1. McCarthy AM, Kontos D, Synnestvedt M, et al. Screening outcomes following implementation of digital breast tomosynthesis in a general-population screening program. J Natl Cancer Inst 2014; 106
2. Lehman CD, White E, Peacock S, Drucker MJ, Urban N. Effect of age and breast density on screening mammograms with false-positive findings. AJR Am J Roentgenol 1999; 173:1651-1655
23. Is annual screening more effective than biennial screening?
Yes. If a woman is going to participate in screening, annual screening is especially important for women in their 40s when cancers tend to be more biologically aggressive. Greater breast density also contributes to worse outcomes from screening mammography among women in their 40s [1, 2]. Biennial screening is nearly as effective as annual screening at reducing deaths due to breast cancer among women who are over the age of 50 (or postmenopausal).
References Cited
1. Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40-49 years old. AJR Am J Roentgenol 2014; 203:1379-1381
2. Bailey SL, Sigal BM, Plevritis SK. A simulation model investigating the impact of tumor volume doubling time and mammographic tumor detectability on screening outcomes in women aged 40-49 years. J Natl Cancer Inst 2010; 102:1263-1271
24. Are there some cancers found by screening mammography that do not require treatment?
Probably, but it is difficult to determine this at the individual level, i.e. for a given patient. Some cancers are so indolent and slow-growing that they might not ever have been detected otherwise in a patient’s lifetime (“overdiagnosis”). While estimates of overdiagnosis vary, on average, of 11 breast cancers found with screening, 2 will be life saving, 1 will represent overdiagnosis, and 8 will be found earlier than they would have been without screening (with better prognosis) [1]. Some ductal carcinoma in situ found on the first screening examination represents overdiagnosis (estimated at 37% of such cases), but new findings on subsequent screens are uncommonly overdiagnosis (estimated at 4% of cancers on annual screens).
References Cited
1. Yen MF, Tabar L, Vitak B, Smith RA, Chen HH, Duffy SW. Quantifying the potential problem of overdiagnosis of ductal carcinoma in situ in breast cancer screening. Eur J Cancer 2003; 39:1746-1754
If a Woman Has Dense Breasts, What Should Be Considered?
25. Does supplemental screening beyond mammography save lives?
Mammography is the only imaging screening modality that has been studied by multiple randomized controlled trials. Across those trials, mammography has been shown to reduce deaths due to breast cancer. The randomized trials that show a benefit from mammography are those in which mammography increased detection of invasive breast cancers before they spread to lymph nodes [1]. No randomized controlled trial has ever been performed on any other imaging screening modality and therefore there are no data showing that supplemental screening will or will not decrease mortality, though it is expected that other screening tests which increase detection of node-negative invasive breast cancers beyond mammography should further reduce breast cancer mortality.
Proving the mortality benefit of any supplemental screening modality would require a very large, very expensive randomized control trial with 15-20 years of follow-up. Given the speed of technological developments, any results would likely be obsolete by the trial’s conclusion. We do know that high-risk women having annual MRI screening are less likely to have advanced breast cancer than their counterparts who were not screened with MRI [2]. We also know that average-risk women who are screened with ultrasound in addition to mammography are unlikely to have palpable cancer in the interval between screens [3, 4] with the rates of such “interval cancers” similar to women with fatty breasts screened only with mammography. The cancers found only on MRI or ultrasound are mostly small invasive cancers (average size of about 1 cm), and the vast majority are node negative [5, 6]; MRI also finds some DCIS. These results suggest there is a benefit to finding additional cancers with supplemental screening, though it is certainly possible that, like mammography, some of the cancers found with supplemental screening are slow growing and may never cause a woman harm, even if left untreated.
References Cited
1. Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: What have we learned? Radiol Clin North Am 2004; 42:793-806, v
2. Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol 2011; 29:1664-1669
3. Corsetti V, Houssami N, Ghirardi M, et al. Evidence of the effect of adjunct ultrasound screening in women with mammography-negative dense breasts: Interval breast cancers at 1 year follow-up. Eur J Cancer 2011; 47:1021-1026
4. Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012; 307:1394-1404
5. Berg WA. Tailored supplemental screening for breast cancer: What now and what next? AJR Am J Roentgenol 2009; 192:390-399
6. Brem RF, Lenihan MJ, Lieberman J, Torrente J. Screening breast ultrasound: Past, present, and future. AJR Am J Roentgenol 2015; 204:234-240
26. Should a routine annual mammogram for a woman with dense breasts be scheduled as a “diagnostic” or a “screening” mammogram?
Screening. “Diagnostic” mammography is monitored by the radiologist during the appointment and “screening” mammography is not. Indications for diagnostic mammography, rather than screening, include, but are not limited to, signs and symptoms of breast cancer such as a lump, bloody or spontaneous clear nipple discharge, skin or nipple retraction. If additional targeted imaging or follow-up is needed for an abnormality seen on the most recent prior breast imaging, a “diagnostic” appointment is also appropriate. In diagnostic breast imaging, additional views or ultrasound may be performed at the same visit if they are needed. The radiologist will interpret the breast imaging during the examination and the woman will leave with her results after a diagnostic mammogram. Women with a personal history of breast cancer can have their routine annual mammograms performed as diagnostic or screening examinations at many facilities. Diagnostic mammography is typically covered by insurance but subject to deductible and copay.
“Screening” mammography is fully covered by insurance under the Affordable Care Act for women over the age of 40 in the United States and may be covered for younger women, if recommended by her physician, depending on the insurance policy. Typically, screening mammograms are interpreted in a quiet, uninterrupted environment with the full benefit of prior examinations. Cancers are better detected and fewer unnecessary additional views (with associated radiation exposure) are recommended in the screening setting. Results are usually sent by mail to the patient within a few days to a week (by law not later than 30 days) after the appointment.
27. If a patient has dense breasts, what additional screening tests are available after a mammogram?
Depending on the patient’s age, risk level (for further explanation see section on Risk Assessment Tools) and breast density, additional screening tools, such as ultrasound or MRI, may be recommended in addition to mammography. The addition of another imaging tool after a mammogram will find more cancers than mammography/tomosynthesis alone (see Table: Summary of Cancer Detection Rates by Screening Method).
It is important to reassure the patient that it is normal for any screening test to find things that may need to be looked at more closely by use of additional testing. While some of these additional findings may be cancerous, the vast majority will not (this is known as a “false positive”); the only way to determine the importance of such findings is through additional imaging and sometimes biopsy.
Both 2D and 3D mammograms are x-ray technologies. X-rays have difficulty penetrating dense tissue and, as such, are more effective in fatty breasts than in extremely dense breasts. Early results suggest that ultrasound finds additional cancers hidden by dense tissue even after 3D mammography, though further study is warranted.
Ultrasound: Ultrasound is the only screening test suggested specifically for women with dense breasts as a supplement to mammography. In dense tissue, physician-performed or technologist-performed ultrasound has been shown to find an additional 2-4 cancers per 1000 women already screened by 2D or 3D/tomosynthesis mammography. Automated whole breast ultrasound, using special equipment, results in detection of another 2-3 cancers per 1000 women screened. Like all screening tools, ultrasound also detects many findings that are not cancer, but that may require follow-up imaging and/or biopsy. There is no x-ray radiation from ultrasound.
MRI: Contrast-Enhanced Magnetic Resonance Imaging (MRI) can find the most breast cancers of any imaging test currently in widespread use. Breast MRI reveals an average of 10 additional cancers per 1000 women screened after mammography, even when both mammography and ultrasound have been performed. The cancer-detection benefit is seen across all breast density categories.
A woman at very high risk for breast cancer (due to a known or suspected mutation in a breast cancer causing gene, or due to a greater than 20% lifetime risk for breast cancer according to the Claus, Tyrer-Cuzick, or other model that predicts risk for pathogenic BRCA mutation [1]) may be eligible to begin screening at age 25, or at least by age 30. In high-risk women, MRI is recommended annually, in addition to mammography, regardless of breast density, though before age 30 sometimes only MRI is performed due to the radiation sensitivity of younger breast tissue. Annual screening MRI is also recommended in women who have had prior radiation therapy to the chest at least 8 years earlier and before age 30, such as for Hodgkin’s lymphoma. Recently, the American College of Radiology recommended annual screening MRI also in women with a personal history of breast cancer diagnosed by age 50, and in women diagnosed later who have dense breasts [2]. Annual supplemental screening MRI can also be considered in women who have a personal history of atypical or risk lesions, such as lobular carcinoma in situ.
MRI of the breasts requires intravenous injection of gadolinium-based contrast and lying in a tunnel-like space that may be difficult for women with claustrophobia. There is no x-ray radiation from MRI. Gadolinium has been shown to accumulate in parts of the brain, but no adverse effects have been shown from this.
MRIs have many false positives (when additional testing or biopsy is recommended for a finding which is not cancer). The benefits and risks of MRI in women who are not at high risk are being studied. In most centers, MRI is a very expensive imaging test that is not covered by insurance unless a woman meets high-risk definitions, and a copay and/or deductible may be incurred. MRI cannot be performed in women with poor kidney function, pacemakers, or certain other metal implants.
The addition of screening ultrasound is usually only recommended in women with dense breasts. Screening MRI is used in high-risk women of all breast densities. If screening MRI is performed, there is no need for screening ultrasound.
Screening MBI, BSGI and Contrast-Enhanced Mammography may be offered to women with dense breasts or as an alternative to MRI at some centers. Further validation of such approaches is in progress.
References Cited
1. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007; 57:75-89
2. Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: Recommendations from the ACR. J Am Coll Radiol 2018; 15:408-414
28. Will insurance cover supplemental screening beyond mammography?
In Europe, national breast screening programs for women of average risk are offered free in nearly all European countries adhering to standards laid out in the European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis. Variations do exist on the ages and risk factors of women invited to participate in routine screening, screening intervals, coverage and supplemental screening modalities utilized. Opportunistic mammography exists in some countries either as the sole screening system or in addition to the national breast screening program. Part of the cost is out of pocket payment or reimbursed by private insurance.
In the U.S., the answer depends on the type of screening, patient’s insurance, risk factors, the state you practice in, and whether or not a law is in effect requiring insurance coverage for additional screening. In Illinois, for example, if ordered by a physician, a woman with dense breasts can receive an ultrasound without a copay or deductible. In New York as of January 1, 2017 all supplemental screening and diagnostic breast imaging are required to be fully covered (no copay/no deductible), though exceptions do exist. Generally, in other states, an ultrasound will be covered if ordered by a physician – but is subject to the copay and deductible of an individual health plan. In New Jersey, insurance coverage is provided for additional testing if a woman has extremely dense breasts. An MRI will generally be covered if the patient meets “high-risk” criteria*. In Michigan, at least one insurance company will cover a screening MRI for normal-risk women with dense breasts at a cost which matches the copay and deductible of a screening mammogram (which in most cases is zero). It is important for the patient to check with her insurance carrier prior to having an MRI.
*For more information on high-risk criteria, see American Cancer Society guidelines: http://www.cancer.org/cancer/breastcancer/moreinformation/breastcancerearlydetection/breast-cancer-early-detection-acs-recs
29. Does 3D mammography (tomosynthesis) solve the problem of screening dense breasts; is there a benefit to screening ultrasound after 3D?
Compared to standard digital mammography, 3D mammography (tomosynthesis) does improve the chance of finding cancer in most breasts. However, in women with extremely dense breasts, studies have shown mixed results as to whether 3D mammograms find more cancers than 2D mammograms. 3D does reduce the chance of having to return for additional diagnostic imaging for a false positive finding (false alarm).
Studies indicate there is benefit from screening ultrasound even after 3D mammography. Four large-scale studies [1-4] showed that ultrasound significantly improved detection of cancer even after the combination of 2D and 3D mammography in dense breasts. However, on average, ultrasound will also show more areas which need follow-up than does mammography. Some of those “finds” will be cancer, but the vast majority of these additional findings, determined after further imaging or biopsy, will be false positives.
References Cited
1. Tagliafico AS, Mariscotti G, Valdora F, et al. A prospective comparative trial of adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts (ASTOUND-2). Eur J Cancer 2018; 104:39-46
2. Destounis S, Arieno A, Morgan R. Comparison of cancers detected by screening ultrasound and digital breast tomosynthesis. Abstract 3162. In: American Roentgen Ray Society (ARRS). New Orleans, LA, 2017
3. Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in mammography-negative dense breasts (ASTOUND): Interim report of a prospective comparative trial. J Clin Oncol 2016
4. Dibble EH, Singer TM, Jimoh N, Baird GL, Lourenco AP. Dense breast ultrasound screening after digital mammography versus after digital breast tomosynthesis. AJR Am J Roentgenol 2019; 213:1397-1402
30. If 3D mammography (tomosynthesis) is performed, will a patient also need a screening ultrasound or MRI?
If a patient has dense breasts, the answer is yes. In several large studies [1-4], including the ASTOUND [1] prospective multicenter trial in Italy, ultrasound significantly improved detection of cancer even after tomosynthesis (3D mammography) or the combination of 2D and 3D mammography in women with dense breasts. A 2020 prospective multicenter study [5] of abbreviated (“fast” or “mini”) MRI in 1444 women with dense breasts found an overall 3D mammography cancer detection rate of 6.2/1000 women screened vs. an overall abbreviated MRI cancer detection rate of 15.2/1000, a difference of 9/1000. If a patient has been recommended to have MRI screening because of her risk factors, she would still have MRI even if tomosynthesis is performed, regardless of her breast density. If screening MRI is performed, then screening ultrasound is not needed.
References Cited
1. Tagliafico AS, Calabrese M, Mariscotti G, et al. Adjunct screening with tomosynthesis or ultrasound in mammography-negative dense breasts (ASTOUND): Interim report of a prospective comparative trial. J Clin Oncol 2016
2. Tagliafico AS, Mariscotti G, Valdora F, et al. A prospective comparative trial of adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts (ASTOUND-2). Eur J Cancer 2018; 104:39-46
3. Destounis S, Arieno A, Morgan R. Comparison of cancers detected by screening ultrasound and digital breast tomosynthesis. Abstract 3162. In: American Roentgen Ray Society (ARRS). New Orleans, LA, 2017
4. Dibble EH, Singer TM, Jimoh N, Baird GL, Lourenco AP. Dense breast ultrasound screening after digital mammography versus after digital breast tomosynthesis. AJR Am J Roentgenol 2019; 213:1397-1402
5. Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. Jama 2020; 323:746-756
31. How do I identify women who are at higher risk who should have MRI?
The American College of Radiology (ACR [1]) recommends all women, but especially Black women and women of Ashkenazi Jewish descent, undergo risk assessment and possible genetic testing by age 30 to identify those at higher risk who can then be counseled to begin earlier and more aggressive screening for breast cancer. All women benefit most from starting screening at least by age 40. In a recent analysis [2] from Harvard, Black, Hispanic, and Asian women have peak incidence of breast cancer in their 40s: it is especially important to start screening at least by age 40 in these groups.
The ACR, National Comprehensive Cancer Network (NCCN) [3], and American Society of Breast Surgeons, recommend annual MRI in the following subgroups of women:
- Women with known disease-causing BRCA1/2 mutation(s), other disease-causing mutation(s), or their untested first-degree relatives, should begin annual screening MRI only between age 25-29, adding annual digital mammography/tomosynthesis at age 30 and beyond.
Note: There is emerging evidence that the benefit of mammography is relatively small in BRCA1 carriers prior to the age of 40; therefore, the ACR suggests BRCA1 mutation carriers may consider delaying mammography until age 40 only if they receive contrast-enhanced MRI annually starting at age 25. Annual mammography is of benefit in those with BRCA2 or other disease-causing mutations. - Women with prior chest/mantle radiation therapy (cumulative dose of ≥ 10 Gy) before age 30 should begin MRI and annual mammography at age 25 or at least 8 years after completion of radiation, whichever is latest.
- Women with a calculated lifetime risk of breast cancer of ≥ 20%. Only models that include detailed family history such as Tyrer-Cuzick (which now includes breast density as a risk factor), BRCAPRO, BOADICEA, Claus, or Penn II, but not the Gail model, should be used to calculate risk for the purposes of MRI screening.
- Women with a personal history of breast cancer and dense breasts or diagnosis by age 50 regardless of breast density. A personal history of breast cancer is not included in risk models, but all women diagnosed with breast cancer at or before age 50 and treated with breast-conserving therapy have a ≥ 20% lifetime risk for a new breast cancer.
- Annual MRI may be considered in addition to annual mammography (with or without tomosynthesis) in women with a personal history of breast cancer diagnosed after age 50 and without dense breasts, and/or a history of lobular carcinoma in situ (LCIS) or prior atypia [e.g. atypical ductal hyperplasia (ADH), or atypical lobular hyperplasia (ALH), or atypical papilloma].
In women who meet guidelines for MRI screening but are unable to tolerate it and who have dense breasts, the ACR suggests ultrasound be considered in addition to annual mammography. If MRI is performed, there is no benefit to screening ultrasound.
Where available, contrast-enhanced mammography may be a reasonable alternative to MRI for high-risk women, though further validation is warranted [4-6].
References Cited
1. Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: Recommendations from the ACR.J Am Coll Radiol2018; 15:408-414
2. Stapleton SM, Oseni TO, Bababekov YJ, Hung YC, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States.JAMA Surg2018; 153:594-595
3. National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2020). https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed December 26, 2020.
4. Xiang W, Rao H, Zhou L. A meta-analysis of contrast-enhanced spectral mammography versus MRI in the diagnosis of breast cancer. Thorac Cancer 2020; 11(6):1423-1432
5. Sorin V, Yagil Y, Yosepovich A, et al. Contrast-Enhanced Spectral Mammography in Women With Intermediate Breast Cancer Risk and Dense Breasts. AJR Am J Roentgenol 2018; 211(5):W267-W274
6. Sung JS, Lebron L, Keating D, et al. Performance of Dual-Energy Contrast enhanced Digital Mammography for Screening Women at Increased Risk of Breast Cancer. Radiology 2019; 293(1):81-88
32. Is gadolinium contrast used in MRI imaging safe?
Gadolinium, a heavy metal, is injected intravenously as a contrast agent to help see breast cancer on MRI examinations. Most of the gadolinium is cleared by the kidneys. There are data showing that small amounts of gadolinium can accumulate in parts of the brain, especially after multiple MRI examinations. The importance of this finding is unknown and has not been linked to any known negative health effects in patients with normal kidney function. There appears to be more gadolinium retained by the body with certain types of contrast agents (so-called “linear” compounds) than with those that are more like a cage around the gadolinium (so-called “macrocyclic” compounds). When kidney function is less than normal, the dose of contrast used may be reduced. Gadolinium contrast should not be used in a patient who is pregnant or who may be pregnant. The Food and Drug Administration has concluded that the benefit of all approved gadolinium-based contrast agents far outweighs any hypothetical risks. Experts on our Medical Advisory Board agree, and we continue to recommend annual contrast-enhanced MRI for women at high risk. For further information, please see: https://www.fda.gov/Drugs/DrugSafety/ucm589213.htm.
33. If recommended to have additional screening with ultrasound or MRI, will a patient need to have that every year?
Usually the answer is yes, though age and other medical conditions will change a patient’s personal risk and benefit considerations and therefore screening recommendations may change from one year to the next. Technology is changing and guidelines also evolve which influence recommendations.
34. Is MBI recommended for screening dense breasts?
Molecular Breast Imaging (MBI) is a specialized nuclear medicine breast imaging technique that requires intravenous injection of a radiopharmaceutical, typically 99mTc-sestamibi. Sestamibi has been in common use as a tracer for nuclear cardiology studies for over 30 years and has an extremely low risk of adverse reactions and no contraindications. Low-dose molecular breast imaging has been used with excellent results by Mayo Clinic and a few other centers for screening women with dense breasts, showing another 7 to 8 cancers after a normal mammogram for every thousand women screened [1-3].
MBI can also be useful as a diagnostic tool in women who have dense breasts and symptoms such as a lump or vague abnormality on mammography that in rare cases cannot be sorted out with additional views or ultrasound. MBI can also be helpful for some women who need but cannot have an MRI [4]. As of the most recent review in 2017, the American College of Radiology Practice Parameter for Molecular Breast Imaging [5] suggests MBI is a potential option for supplemental screening in high-risk women and those with dense breasts who cannot undergo MRI, but it is usually not indicated, as the technique involves ionizing radiation to the whole body with attendant risk of potentially inducing cancer [6]. MBI is never used in women who are pregnant.
The radiation exposure from low-dose MBI, performed with a delivered dose of 6 to 8 mCi 99mTc-sestamibi, is higher than that from a mammogram. Further, mammography delivers radiation to the breast only, while MBI delivers radiation to the whole body. In order to compare radiation doses from these different types of exams, a standard calculation called “effective radiation dose” is used, which takes into account which body parts are exposed to radiation by a given test and how sensitive every exposed organ is to radiation. Effective dose has units of milli-Sieverts (mSv). The effective dose of mammography is about 0.5 mSv and the effective dose from a low-dose MBI is about 1.8 to 2.4 mSv. For comparison, the radiation dose received from normal daily life is between 3 and 10 mSv per year, depending on where you live [7, 8]. Below effective doses of 100 mSv, no health risks from radiation have been proven according to national and international radiation physics experts [9-11].
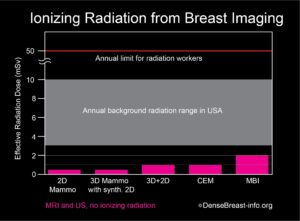
Chart 1. This graph shows the whole body effective radiation doses from common breast screening exams: 2D mammography; 3D (tomosynthesis) mammography with synthetic 2D; 2D combined with 3D mammography; contrast-enhanced mammography (CEM); and molecular breast imaging (MBI). MBI, with the highest dose, at 2 mSv, is below the range of annual background radiation exposure in the USA. The annual limit for radiation workers is 50 mSv. At doses below 100 mSv, no risks have been proven, and benefits to patients outweigh any possible risks. Any risk from radiation is greater in younger individuals, especially those under the age of 30, and radiation exposure should always be minimized (except when undergoing cancer treatment).
References Cited
1. Rhodes DJ, Hruska CB, Phillips SW, Whaley DH, O’Connor MK. Dedicated dual-head gamma imaging for breast cancer screening in women with mammographically dense breasts. Radiology2011; 258:106-118
2. Rhodes DJ, Hruska CB, Conners AL, et al. JOURNAL CLUB: Molecular breast imaging at reduced radiation dose for supplemental screening in mammographically dense breasts. AJR Am J Roentgenol2015; 204:241-251
3. Shermis RB, Wilson KD, Doyle MT, et al. Supplemental breast cancer screening with molecular breast imaging for women with dense breast tissue. AJR Am J Roentgenol2016:1-8
4. NCCN Guidelines. Breast cancer screening and diagnosis v.1.2023. National Comprehensive Cancer Network. June 19, 2023. Acessed August 3, 2023. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf
5. ACR practice parameter for the performance of molecular breast imaging (MBI) using a dedicated gamma camera. American College Of Radiology. Revised 2022. Accessed August 2, 2023. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/MBI.pdf
6. ACR appropriateness criteria: Breast cancer screening. American College Of Radiology. Revised 2017. Accessed August 2, 2023. https://acsearch.acr.org/docs/70910/Narrative/
7. Radiation Sources and Doses. EPA United States Environmental Protection Agency. Updated February 16, 2023. Accessed August 2, 2023. https://www.epa.gov/radiation/radiation-sources-and-doses
8. Biological Effects of Radiation Fact Sheet. United States Nuclear Regulatory Commission. December 2004. Accessed August 2, 2023. https://www.nrc.gov/docs/ML0715/ML071570056.pdf
9. Radiation risk in perspective: Position statement of the Health Physics Society. Health Physics Society. January 1996. Updated February 2019. Accessed August 2, 2023. https://hps.org/documents/radiationrisk.pdf
10. AAPM position statement on radiation risks from medical imaging procedures, Policy number PS 4-A. American Association of Physicists in Medicine. April 10, 2018. Accessed August 2, 2023. https://www.aapm.org/org/policies/details.asp?id=318&type=PP¤t=true
11. Biological Mechanisms of radiation actions at low doses: A white paper to guide the Scientific Committee’s future programme of work. Radiation UNSCotEoA. 2012; Accessed August 2, 2023. https://www.unscear.org/docs/reports/Biological_mechanisms_WP_12-57831.pdf
35. Should thermography be used to screen dense breasts?
No. According to the FDA, “There is no valid scientific data to demonstrate that thermography devices, when used on their own or with another diagnostic test, are an effective screening tool for any medical condition including the early detection of breast cancer or other diseases and health conditions [1].”
Thermography is a non-invasive technique that uses infrared technology to detect both heat and blood flow patterns very near the skin’s surface. Although some large cancers can be seen, these are usually palpable and so thermography adds little. Thermography has a high “false negative” rate (when a test result indicates “no cancer” – though cancer is actually present), especially for small breast cancers, so it does not play a role in screening asymptomatic women. Thermography also has a high rate of “indeterminate” findings, which on additional diagnostic evaluation (mammography, ultrasound, MRI, follow-up observation, etc.) indicate no cancer is found. These often prompt a recommendation for short interval follow-up, which creates anxiety and additional cost to the woman.
References Cited
1. US Food and Drug Administration. FDA warns thermography should not be used in place of mammography to detect, diagnose, or screen for breast cancer: FDA Safety Communication. https://www.fda.gov/consumers/consumer-updates/breast-cancer-screening-thermogram-no-substitute-mammogram Published February 25, 2019. Accessed May 18, 2020
36. Can the decision on supplemental screening this year be based on patient’s breast density last year?
The answer is essentially “yes”. At the population level, there is a tendency for slight decrease in breast density each year, and this tends to be more abrupt in the few years around menopause. One study [1] showed that only 7% of women who were considered not dense one year were classified as “dense” the following year; similarly 6% of women considered “dense” one year were classified as not dense the following year. For 87% of women, there was no change from one year to the next. Any difference that might affect the decision for supplemental screening would be between women considered to have heterogeneously dense or scattered fibroglandular density one year or the other, and radiologists may differ in this assessment even when there is no true change in the breasts. In a patient with breast density near the threshold, there are likely to be areas in the breast where cancer could be masked: it is not unreasonable to have had supplemental screening even if one’s breasts turn out to be slightly less dense this year.
References Cited
1. Cohen SL, Margolies LR, Schwager SJ, et al. Early discussion of breast density and supplemental breast cancer screening: Is it possible? Breast J 2014; 20:229-234
37. What is Dedicated Breast PET (Positron Emission Mammography, PEM)?
What is it?
Breast PET uses an injection of a short-lived radioactive sugar (18FDG) into the body to detect metabolically active lesions such as cancer. PET, or more often PET-CT, is commonly used to stage the whole body in patients with larger breast cancers or suspected recurrence. Newer technologies can provide detailed PET images of the breasts to assess local extent of breast cancer.
How it works
The radioactive sugar accumulates in cancer cells in the breast and emits high-energy positron radiation that is detected and analyzed. For one such system, the patient is seated and the breast is gently stabilized; positioning is otherwise like mammography (Fig. PET-1).


Benefits
Breast PET is generally considered a diagnostic test used to determine the extent of cancer within the breasts, and can be used as an alternative to breast MRI for that purpose [1, 2]. In women with newly diagnosed breast cancer being treated with chemotherapy prior to surgery, breast PET can also help monitor response to treatment. In addition, breast PET may help distinguish recurrence of cancer from scar in women who have been previously treated for breast cancer. Uncommonly, it can be used for problem solving (Fig. PET-3). It is a relatively new modality and not widely available.
Considerations
Breast PET exposes the patient to a moderately high whole body radiation dose and is not used for screening [3]. The very back part of the breast near the chest wall and the axillary lymph nodes are better evaluated with MRI.

References
1. Berg WA, Madsen KS, Schilling K, et al. Breast cancer: Comparative effectiveness of positron emission mammography and MR imaging in presurgical planning for the ipsilateral breast. Radiology 2011; 258:59-72
2. Narayanan D, Berg WA. Use of breast-specific PET scanners and comparison with MR imaging. Magn Reson Imaging Clin N Am 2018; 26:265-272
3. Narayanan D, Berg WA. Dedicated breast gamma camera imaging and breast PET: Current status and future directions. PET Clin 2018; 13:363-381
What Are Factors That May Affect Breast Density?
38. Can breast density be changed with diet? Is fat in the diet related to breast density?
Dietary fat intake has little to do with breast density; however, it does relate to increased body mass index (BMI). BMI is a measure of body fat based on height and weight, and there is more fatty breast tissue in women with higher BMI. Higher BMI reduces the percent of density but might not reduce the total amount of dense tissue. BMI and breast density are both separate risk factors for breast cancer. Before menopause, low BMI [1, 2] increases the risk of breast cancer. After menopause, weight gain and increasing BMI increase the risk of breast cancer.
References Cited
1. van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol 2000; 152:514-527
2. Huo CW, Chew GL, Britt KL, et al. Mammographic density-a review on the current understanding of its association with breast cancer. Breast Cancer Res Treat 2014; 144:479-502
39. Can exercise affect a woman’s breast density category?
It can. While exercise can decrease the amount of fat in the breast, the glandular or dense breast tissue is not affected by exercise. So, if a woman loses a lot of weight due to exercise, her breasts can appear more dense due to loss of fat (the amount of fat decreases while the amount of dense tissue remains the same) [1].
While exercise does not decrease the actual amount of dense tissue in the breasts, it does decrease the overall risk of developing breast cancer [2].
References Cited
1. Peters TM, Ekelund U, Leitzmann M, et al. Physical activity and mammographic breast density in the EPIC-Norfolk cohort study. Am J Epidemiol 2008; 167:579-585
2. Azam S, Kemp Jacobsen K, Aro AR, et al. Regular physical activity and mammographic density: a cohort study. Cancer Causes & Control 2018; 29:1015-1025
40. Does taking hormone replacement therapy (HRT) or estrogen therapy alone affect breast density or breast cancer risk?
- Increases in both breast density [1, 2] and breast cancer risk [3, 4] have been shown in patients taking oral or transdermal combined estrogen and progesterone hormone supplements (i.e. hormone replacement therapy, HRT).
- In women who have had breast cancer, oral or transdermal HRT have been shown to increase risk of breast cancer recurrence [5].
- Data are conflicting as to whether taking oral or transdermal estrogen alone in women who have had a hysterectomy increases breast density [5-9] or breast cancer risk [3, 4].
- There are minimal data on the effect of vaginal estrogen on breast density.
- In women who have not had breast cancer, vaginal estrogen has not been shown to increase breast cancer risk.
- In women who have had breast cancer, vaginal estrogen has not been shown to increase breast cancer recurrence, except in one study that reported an increased risk in breast cancer survivors who took aromatase inhibitors [6]. Vaginal estrogen has not been shown to increase death from breast cancer in breast cancer survivors [6, 13].
A large meta-analysis of prospective observational studies found that ever users of HRT had a 30% greater risk of developing breast cancer than never users [relative risk (RR) 1.29 (95% CI 1.27, 1.30)][3]. Risk increased with duration of use for both current and past users, with higher risk at any given duration of use for current users [current users, RR 1.20 (95% CI 1.01-1.43) for <1 year of use to 2.51 (95% CI 2.35-2.68) for ≥ 15 years of use; past users, RR 1.02 (95% CI 0.95-1.10) for <1 year of use to 1.30 (95% CI 1.25-1.37) for ≥ 15 years of use]. Similarly, the Women’s Health Initiative randomized trial found nearly 30% greater risk after a median 20 years of follow-up among women randomized to estrogen and medroxyprogesterone acetate (hazard ratio 1.28; 95% CI 1.13-1.45) [4].
Data are conflicting as to whether taking oral or transdermal estrogen alone in women who have had a hysterectomy increases breast density [7-11] or breast cancer risk [3, 4]. There are minimal data on the effect of vaginal estrogen on breast density. Vaginal estrogen has not been shown to increase breast cancer risk [3, 12] in women who have not had breast cancer.
In women who have had breast cancer, oral or transdermal HRT have been shown to increase risk of breast cancer recurrence [5]. Vaginal estrogen has not been shown to increase risk of breast cancer recurrence, except in one study that reported an increased risk in breast cancer survivors who used vaginal estrogen and took aromatase inhibitors [6]. Vaginal estrogen has not been shown to increase death from breast cancer in women who have had breast cancer [6, 13].
References Cited
1. Azam S, Jacobsen KK, Aro AR, Lynge E, Andersen ZJ. Hormone replacement therapy and mammographic density: a systematic literature review. Breast Cancer Res Treat 2020;182(3):555-579.
2. McTiernan A, Martin CF, Peck JD, et al.; Women’s Health Initiative Mammogram Density Study Investigators. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women’s Health Initiative randomized trial. J Natl Cancer Inst 2005;97(18):1366-76.
3. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet 2019 28;394(10204):1159-1168.
4. Chlebowski RT, Anderson GL, Aragaki AK, et al. Association of Menopausal Hormone Therapy With Breast Cancer Incidence and Mortality During Long-term Follow-up of the Women’s Health Initiative Randomized Clinical Trials. JAMA 2020;324(4):369-380.
5. Poggio F, Del Mastro L, Bruzzone M, et al. Safety of systemic hormone replacement therapy in breast cancer survivors: a systematic review and meta-analysis. Breast Cancer Res Treat 2022;191(2):269-275.
6. Cold S, Cold F, Jensen MB, Cronin-Fenton D, Christiansen P, Ejlertsen B. Systemic or Vaginal Hormone Therapy After Early Breast Cancer: A Danish Observational Cohort Study. J Natl Cancer Inst 2022;114(10):1347-1354.
7. Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst 2003;95(1):30-7.
8. Grady D, Vittinghoff E, Lin F, et al. Effect of ultra-low-dose transdermal estradiol on breast density in postmenopausal women. Menopause 2007;14:391-396.
9. Maskarinec G, Pagano I, Lurie G, Kolonel LN. A longitudinal investigation of mammographic density: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2006;15(4):732-9.
10. McTiernan A, Chlebowski RT, Martin C, et al. Conjugated equine estrogen influence on mammographic density in postmenopausal women in a substudy of the women’s health initiative randomized trial. J Clin Oncol 2009;20;27(36):6135-43.
11. Aiello EJ, Buist DS, White E. Do breast cancer risk factors modify the association between hormone therapy and mammographic breast density? (United States). Cancer Causes Control 2006;17(10):1227-35.
12. Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause 2018 Jan;25(1):11-20.
13. McVicker L, Labeit AM, Coupland CAC, et al. Vaginal Estrogen Therapy Use and Survival in Females With Breast Cancer. JAMA Oncol 2024;10(1):103-108.
41. Is there anything a patient can do to decrease her breast density? What about taking tamoxifen?
Tamoxifen blocks the estrogen receptor in breast cells and in breast cancer cells which express the estrogen receptor. Tamoxifen may be recommended to reduce the risk of developing breast cancer in women who have had prior atypical biopsies. Tamoxifen is also prescribed for women who have had breast cancer that expresses estrogen receptors to decrease recurrence. One study [1] found that when breast density is carefully measured by computer software, women whose breasts became at least 10% less dense while taking tamoxifen had a 63% reduction in risk of developing breast cancer – whereas those whose breast density did not change did not see a decrease in their risk. Several similar studies [2, 3] in women who have had breast cancer showed that only women whose breast density decreased on tamoxifen had decreased risk of recurrence. Tamoxifen also carries about a 3% risk of blood clots (which could result in pulmonary embolism or stroke) and a smaller risk of endometrial cancer (if the woman still has her uterus).
If a woman is on hormone therapy for menopausal symptoms, her breast density may decrease if she stops taking hormone supplements.
References Cited
1. Cuzick J. Breast density predicts endocrine treatment outcome in the adjuvant setting. Breast Cancer Res 2012; 14:109
2. Cuzick J, Warwick J, Pinney E, et al. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case-control study. J Natl Cancer Inst 2011; 103:744-752
3. Li J, Humphreys K, Eriksson L, Edgren G, Czene K, Hall P. Mammographic density reduction is a prognostic marker of response to adjuvant tamoxifen therapy in postmenopausal patients with breast cancer. J Clin Oncol 2013; 31:2249-2256
42. Will taking Arimidex or other aromatase inhibitors affect breast density?
Aromatase inhibitors block the body’s own production of estrogen and are prescribed for postmenopausal women who have had breast cancer where the tumor cells express receptors for estrogen. One study [1, 2] looked at women who have had breast cancer. When breast density is carefully measured by computer software, women who experienced a decrease in breast density while taking tamoxifen or aromatase inhibitors had a lower risk of recurrence than women who did not experience a decrease in breast density.
References Cited
1. Kim J, Han W, Moon HG, et al. Breast density change as a predictive surrogate for response to adjuvant endocrine therapy in hormone receptor positive breast cancer. Breast Cancer Res 2012; 14:R102
2. Kim J, Han W, Moon HG, et al. Erratum to Breast density change as a predictive surrogate for response to adjuvant endocrine therapy in hormone receptor positive breast cancer. Breast Cancer Res 2012; 14:403
43. For breast cancer survivors, is there a correlation between dense breasts and the likelihood of cancer in the opposite breast?
Yes. There is a 1.8-fold higher risk of cancer in the opposite breast among women with dense breasts [1], but density and associated risk can be reduced with treatment. A 10% decrease of mammographic density or more within the first two years after an original diagnosis, as a result of treatment, is associated with a significantly reduced risk of cancer in the opposite breast (known as contralateral breast cancer) [2]. This potential new risk predictor can thus contribute to decision-making in follow-up treatment – particularly the continuation of a chemoprevention drug, like tamoxifen or aromatase inhibitors, which reduce breast density in some women [3].
References Cited
1. Raghavendra A, Sinha AK, Le-Petross HT, et al. Mammographic breast density is associated with the development of contralateral breast cancer. Cancer 2017; 123:1935-1940
2. Sandberg ME, Li J, Hall P, et al. Change of mammographic density predicts the risk of contralateral breast cancer: a case-control study. Breast Cancer Res 2013; 15:R57
3. Engmann NJ, Scott CG, Jensen MR, et al. Longitudinal changes in volumetric breast density with Tamoxifen and aromatase inhibitors. Cancer Epidemiol Biomarkers Prev 2017; 26:930-937
44. Is breast density an issue that affects men?
Not normally, though rarely men do get breast cancer. Normal male breasts are mostly fatty. Sometimes men’s breasts do become enlarged and develop glandular tissue due to a condition called gynecomastia. This enlargement, due to a hormonal imbalance, normally affects one breast more than the other. If a mammogram is performed, it is usually easy to distinguish gynecomastia from breast cancer.
45. Is breast density inherited?
Breast density is at least partially inherited, though it is complex to predict. If a patient’s mother had dense breast tissue, it is more likely the patient will too.
46. Does the increase in density due to breastfeeding affect the ability of screening tests, like a mammogram, to find breast cancer?
Yes. Changes in the breast during breastfeeding do reduce the accuracy of screening tests (like mammography or MRI). Unless the patient plans to be breastfeeding for more than one to two years and is at high risk, it is generally recommended to wait at least a few months after breastfeeding stops before resuming breast screening. Ultrasound is usually performed first if a patient is breastfeeding and there is a concern about breast symptoms. If a breastfeeding woman needs to undergo breast imaging, it is advisable to nurse or pump immediately before the exam.
47. Do Black women have denser breasts? Do Asian women have denser breasts? Do Hispanic women have denser breasts?
Analysis of over 2.6 million mammogram results from the Breast Cancer Surveillance Consortium confirms greater prevalence of dense breasts in Asian women (66%) than non-Hispanic (NH) White women (45%), Hispanic women (45%), or Black women (37%). Breast density is inversely related to body mass index (BMI) and decreases with age and menopause. After correcting for BMI, age, and menopausal status, Black women were more likely to have dense breasts than NH White or Hispanic women.
References Cited
1. Kerlikowske K, Bissell MCS, Sprague BL, et al. Impact of BMI on Prevalence of Dense Breasts by Race and Ethnicity. Cancer Epidemiol Biomarkers Prev 2023:OF1-OF7
2. del Carmen MG, Halpern EF, Kopans DB, et al. Mammographic breast density and race. AJR Am J Roentgenol 2007; 188:1147-1150
3. McCarthy AM, Keller BM, Pantalone LM, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Inst 2016; 108
48. Are there any special considerations for screening of Black or Hispanic women?
Black women are more likely to have disease-causing mutations in BRCA. The American College of Radiology recommends Black women be evaluated in a high-risk program by age 30, even in the absence of family history [1]. Further, it is more important for Black women and Hispanic women to start screening at least by age 40 as peak breast cancer incidence occurs in the mid 40s, compared to peak in the early 50s for Caucasian women [2].
References Cited
1. Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: Recommendations from the ACR. J Am Coll Radiol 2018; 15:408-414
2. Stapleton SM, Oseni TO, Bababekov YJ, Hung YC, Chang DC. Race/ethnicity and age distribution of breast cancer diagnosis in the United States. JAMA Surg 2018; 153:594-595
49. Is there a relationship between having dense breasts and pathogenic BRCA gene variants (mutations)?
Disease-causing BRCA gene mutations and dense breasts are each independent risk factors for the development of breast cancer; however, pathogenic BRCA1 or BRCA2 mutations are associated with a much higher risk than that of having dense breasts. As BRCA1 and BRCA2 mutations are associated with a higher risk, MRI is part of routine screening beginning at age 25 to 30 for women who have these mutations, regardless of breast density. Cancers are also more likely to develop at a younger age in women with disease-causing mutations in BRCA genes, and the breasts are usually dense at younger ages making mammography especially ineffective as a standalone test in such women.
50. What is the recommended breast screening for women with disease-causing genetic variants (mutations)?
National Comprehensive Cancer Network (NCCN) guidelines recommend annual screening MRI beginning by age 25, with the addition of mammography beginning at age 30, in women who are known to carry pathogenic/likely pathogenic variants in BRCA1 or BRCA2 (unless the woman has had bilateral mastectomy), and in women who are untested first-degree relatives of those with known pathogenic/likely pathogenic variants (see table below) [1].
Women who are known to carry or are first-degree untested relatives of individuals with less common disease-causing variants [such as those associated with Li-Fraumeni syndrome (TP53); Bannayan-Riley-Ruvalcaba syndrome or Cowden syndrome (PTEN); hereditary diffuse gastric cancer (CDH1); Peutz-Jeghers syndrome (STK11); Neurofibromatosis type 1 (NF1); or Fanconi anemia (PALB2)] are also recommended for annual screening MRI, with age to start depending on the variant (see table below). Women with NF1 have elevated risk only through age 50: supplemental MRI should stop beyond age 50 for these women. For women with disease-causing variants in genes other than NF1, supplemental MRI screening should continue until age 75, after which screening should continue on an individual basis [1].
In addition to pathogenic/likely pathogenic variant carriers and their untested first-degree relatives, annual screening MRI is recommended in addition to mammography/tomosynthesis in the following subgroups of women:
- Women with a calculated lifetime risk (LTR) of breast cancer of 20% or greater are recommended to begin annual screening MRI. Starting age varies according to different guidelines [2-5] but may be as early as age 25-30 [4]. Any of the models used to predict risk of a pathogenic variant [Tyrer-Cuzick (IBIS), Penn II, BOADICEA, BRCAPRO], or the Claus model, but NOT the Gail model, can be used to estimate lifetime risk for purposes of annual screening MRI eligibility.
- Women with prior chest radiation therapy (such as for Hodgkin disease) between ages 10 and 30 are at high risk for developing breast cancer [2-6], similar to BRCA1 or BRCA2 carriers, and are recommended for annual screening MRI starting at age 25 or 8 years after the chest radiation therapy, whichever is later.
- Women with a personal history of breast cancer and dense breasts and/or diagnosis by age 50 [3, 4]. All women diagnosed with breast cancer at or before age 50 and treated with breast-conserving therapy have a ≥ 20% risk for a new breast cancer [4].
- Annual MRI may be considered in addition to annual mammography or tomosynthesis in women with a personal history of breast cancer diagnosed after age 50 or without dense breasts [4], and/or a history of LCIS or prior atypia (ADH, ALH, atypical papilloma) [3, 4].
NCCN Breast Cancer Screening Guidelines in Women Who Carry or Are First-Degree Untested Relatives of Individuals with Pathogenic Variants or Variants Known or Likely to Increase Breast Cancer Risk [1]
| Gene | Associated Hereditary Cancer Syndromes | NCCN Breast Cancer Screening Guidelines | |
| Starting age for MRI (yrs)a | Starting age for mammogram (yrs) |
||
| TP53 | Li-Fraumeni syndrome | 20b | 30 |
| BRCA1 | BRCA-related breast and/or ovarian cancer syndrome |
25c | 30 |
| BRCA2 | BRCA-related breast and/or ovarian cancer syndrome |
25c | 30 |
| STK11 | Peutz-Jeghers syndrome | 30 | 30 |
| CDH1 | Hereditary diffuse gastric cancer | 30d | 30d |
| NF1 | Neurofibromatosis type 1 | 30d,e | 30d, |
| PALB2 | 30d | 30d | |
| PTEN | Cowden syndrome/PTEN hamartoma tumor syndrome, Bannayan-Riley-Ruvalcaba syndrome |
35f | 35f |
| ATM | Ataxia telangiectasia (A-T) | 30-35d,g | 40d,g |
| CHEK2 | 30-35d,g | 40d,g | |
| BARD1 | BARD1-related cancer risk (women
only) |
40d,g | 40d,g |
| RAD51C |
40 | 40 | |
| RAD51D |
40 | 40
©DenseBreast-info.org |
|
a For women with pathogenic/likely pathogenic variants who are treated for breast cancer and have not had bilateral mastectomy, screening should continue as described until age 75 (other than for women with NF1f). After age 75, management should be considered on an individual basis [1].
b Start at age 20 or the age of the earliest diagnosed breast cancer in the family if younger than age 20.
c Begin at age 25, or starting age may be individualized based on family history of a breast cancer diagnosis before at 30. From age 25-29 years, breast MRI alone is recommended. Adding mammography at age 30 is preferred due to the potential risk of radiation exposure in pathogenic/likely pathogenic variant carriers under age 30. Mammography can be performed from ages 25-29 years only if MRI is unavailable.
d Start at stated age or 5-10 years before the earliest known breast cancer in the family (whichever comes first), or based on specific pathogenic/likely pathogenic variant.e There are currently no data to suggest an increased breast cancer risk after age 50 years in women with NF1; therefore, MRI screening may discontinue at 50 years of age in this group. In addition, the presence of breast neurofibromas may lead to false-positive MRI results; more data on sensitivity and specificity of MRI in women with NF1 is needed.
f Start at stated age or 10 years before the earliest known breast cancer in the family (whichever comes first).
g The use of MRI in these patients depends on a number of risk factors, including family history, age, breast density, and patient preference.
References Cited
1. National Comprehensive Cancer Network. Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic. (Version 3.2023).https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf. Accessed March 2, 2023.
2. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography.CA Cancer J Clin2007; 57:75-89
3. National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2020).https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed December 26, 2020.
4. Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: Recommendations from the ACR.J Am Coll Radiol2018; 15:408-414
5. Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol 2008; 18:1307-1318
6. Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer.JAMA2009; 301:404-414
51. How do women under the age of 40 find information about breast density and their risks for breast cancer?
Dense breasts are mostly an issue affecting mammography performance so a patient generally does not need to know until they begin having mammograms. For women at normal risk, mammography is often recommended to begin at age 40. If a woman has a family history of breast cancer and has not begun mammography screening, she should speak to her doctor about personal risk factors and when mammography and possibly other screening should begin. As a general guide, if a woman’s mother or sister was diagnosed with breast cancer before age 50, she may want to begin annual screening 10 years before the relative’s age at diagnosis, but not before age 30.
European guidelines recommend double-read biennial screening digital mammography for average-risk women 50 to 69 years of age. Also encouraged are biennial screening for women 73 to 75 years of age, and annual screening for ages 40–45 to 49 [1].
References Cited
1. Sardanelli F, Aase HS, Alvarez M, et al. Position paper on screening for breast cancer by the European Society of Breast Imaging (EUSOBI) and 30 national breast radiology bodies from Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Israel, Lithuania, Moldova, The Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Spain, Sweden, Switzerland and Turkey. Eur Radiol 2017; 27:2737-2743