| QUICK FACTS |
Any additional testing after a mammogram will find more things to be checked. Some may be cancer, but most will not (known as a false positive). To know whether a suspicious finding is cancer or not, further testing and possibly biopsy may be necessary. |
What is it?
Molecular Breast Imaging (MBI) is a specialized nuclear medicine breast imaging technique that requires intravenous injection of a radioactive material and uses a specialized gamma camera imaging system.
Some centers are using MBI for supplemental screening for women with dense breasts. MBI can also be used for further evaluation of abnormalities seen on mammography or breast ultrasound. MBI can be used in women when breast MRI is recommended but cannot be performed. The American College of Radiology indicates MBI is usually not appropriate for screening due to relative lack of evidence and higher radiation exposure [1], though the Society of Nuclear Medicine does endorse its use for screening women with dense breasts [2].
How it works
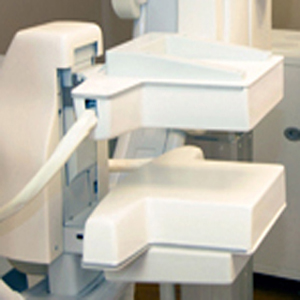
The short-lived radioactive material 99mTc-sestamibi accumulates in cancer cells more than normal cells, and this difference allows cancers to be seen. Starting about 5 minutes after intravenous injection of the radioactive material, each breast is gently stabilized between two detectors, similar to mammographic positioning but with less compression. (MBI-Figure 1), Two views of each breast are taken, and each view takes about 7 to 10 minutes to complete (for a total of 28 to 40 minutes for a routine examination).
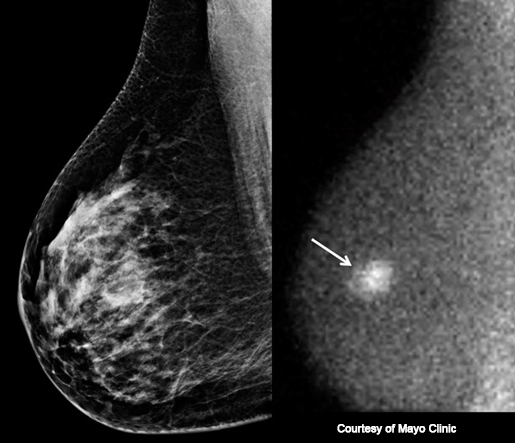
Unlike mammography or ultrasound, MBI does not look at the structure or anatomy of the breast. Instead, MBI reveals functional behavior of breast tissue because the radioactive material accumulates in areas of rapid cell division such as cancers. The radioactive material emits invisible gamma rays, and a gamma camera is used to detect these gamma rays. Areas with more intense accumulation of radioactive material are visible on MBI, even in dense breast tissue, and may represent cancer (see MBI-Figure 2).
Benefits
MBI, performed with a low-radiation-dose protocol, detects an additional 7 to 8 cancers per thousand women screened compared to 2D mammography alone and 6 cancers per thousand women screened with 3D mammography alone, as shown in prospective clinical trials conducted at the Mayo Clinic [3-7]. Similar results have been observed in community practice [5, 6]. In the prospective multicenter Density MATTERS trial, presented at the Radiological Society of North America 2023 annual meeting [7], 2978 women with dense breasts were enrolled for two rounds of annual screening with 3D mammography (tomosynthesis) and MBI. There were 37 women diagnosed with cancer in the first year, with 15 (42%) seen on 3D mammography alone and 34 (92%) seen after combined 3D mammography and MBI, for an added cancer yield from MBI of 6.4/1000 women screened. Of 2587 women completing the second round of screening, 11 women were diagnosed with cancer, including 4 seen on 3D mammography alone and 11 after combined 3D mammography and MBI, for an added cancer yield from MBI of 2.7/1000, with follow-up pending on some of these women. Of a total of 28 cancers seen only on MBI, 21 (75%) were invasive, with median size 0.9 cm. In the first round, 254/2941 (8.6%) women experienced a false-positive recall due to addition of MBI. In the second round, 108/2565 (4.2%) had a false-positive recall due to addition of MBI. To date, there were three cancers missed even after combined 3D mammography and MBI: two DCIS and one 0.8 cm invasive lobular cancer.
MBI can be helpful for women who need but cannot tolerate MRI due to kidney failure, claustrophobia, pacemakers, or other metallic implants [8, 9].
Considerations
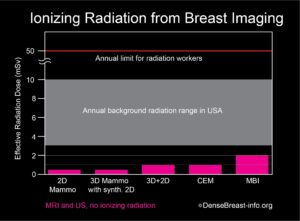
With modern systems, MBI uses about 8 mCi 99mTc-sestamibi, resulting in an effective radiation dose that is about four times that of a mammogram [9]. Most of the radiation dose from sestamibi is deposited in the gastrointestinal tract (gall bladder, colon) and bladder, and not the breasts. The overall radiation exposure from MBI is very small and considered within acceptable limits (Table 1).
Among premenopausal women undergoing MBI, accumulation of the radioactive material can be higher in normal breast tissue during the latter half of the menstrual cycle, which may complicate interpretation of the test. To minimize this issue, premenopausal women undergoing MBI may wish to schedule their test earlier in their menstrual cycle (typically 7 to 14 days after the period starts) when possible. MBI is not used in women who are pregnant.
MBI is more effective at detecting invasive ductal cancer than invasive lobular cancer, with sensitivity of 86% vs. 57% (P<.0001) in the largest published experience from Mayo Clinic [10].
Facilities offering MBI should have direct biopsy capability; otherwise, MRI may be needed to clarify or biopsy an abnormality detected on MBI.
For information about breast imaging insurance laws by state, please visit our Legislation Map here.
Latest update 1/17/24
1. Weinstein SP, Slanetz PJ, Lewin AA, et al. ACR Appropriteness Criteria Supplemental Breast Cancer Screening Based on Breast Density. Available at https://acsearch.acr.org/docs/3158166/Narrative/. American College of Radiology. Accessed May 11, 2021.
2. Hruska CB, Corion C, De Geus-Oei LF, et al. SNMMI Procedure Standard/EANM Practice Guideline for Molecular Breast Imaging with Dedicated y-Cameras. J Nucl Med Technol. 2022; 50 (2) 103-110
3. Rhodes DJ, Hruska CB, Phillips SW, Whaley DH, O’Connor MK. Dedicated dual-head gamma imaging for breast cancer screening in women with mammographically dense breasts. Radiology 2011; 258:106-118
4. Rhodes DJ, Hruska CB, Conners AL, et al. JOURNAL CLUB: Molecular breast imaging at reduced radiation dose for supplemental screening in mammographically dense breasts. AJR Am J Roentgenol 2015; 204:241-251
5. Shermis RB, Wilson KD, Doyle MT, et al. Supplemental breast cancer screening with molecular breast imaging for women with dense breast tissue. AJR Am J Roentgenol 2016:1-8
6. Hruska CB, O’Connor MK. Curies, and grays, and sieverts, oh my: A guide for discussing radiation dose and risk of molecular breast imaging. J Am Coll Radiol 2015; 12:1103-1105
7. Hruska CB, Hunt K, Miller P, et al. Molecular Breast Imaging for Women with Dense Breasts: Results Update from the Density MATTERS Trial. Presented at Radiologic Society of North America Annual Meeting, November 27, 2023, Chicago, IL
8. National Comprehensive Cancer Network. NCCN Guidelines. Breast cancer screening and diagnosis v.3.2023. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast- screening.pdf. Accessed January 15, 2024.
9. Hruska CB. Molecular breast imaging for screening in dense breasts: State of the art and future directions. AJR Am J Roentgenol 2017; 208:275-283
10. Conners AL, Jones KN, Hruska CB, Geske JR, Boughey JC, Rhodes DJ. Direct-Conversion Molecular Breast Imaging of Invasive Breast Cancer: Imaging Features, Extent of Invasive Disease, and Comparison Between Invasive Ductal and Lobular Histology. AJR Am J Roentgenol 2015; 205:W374-W381
